mRNA technologies: Looking beyond COVID vaccines
Following the COVID-19 pandemic, mRNA-based vaccines have been shown to provide rapid and precise immune responses, however, this technology has much wider applications.
Many leading pharma and biotechs have invested in messenger RNA (mRNA) technology to develop COVID-19 vaccines and are now exploring their potential in other disease areas (Figure 1).
Table 1: Leading companies developing mRNA-based vaccines and therapeutics
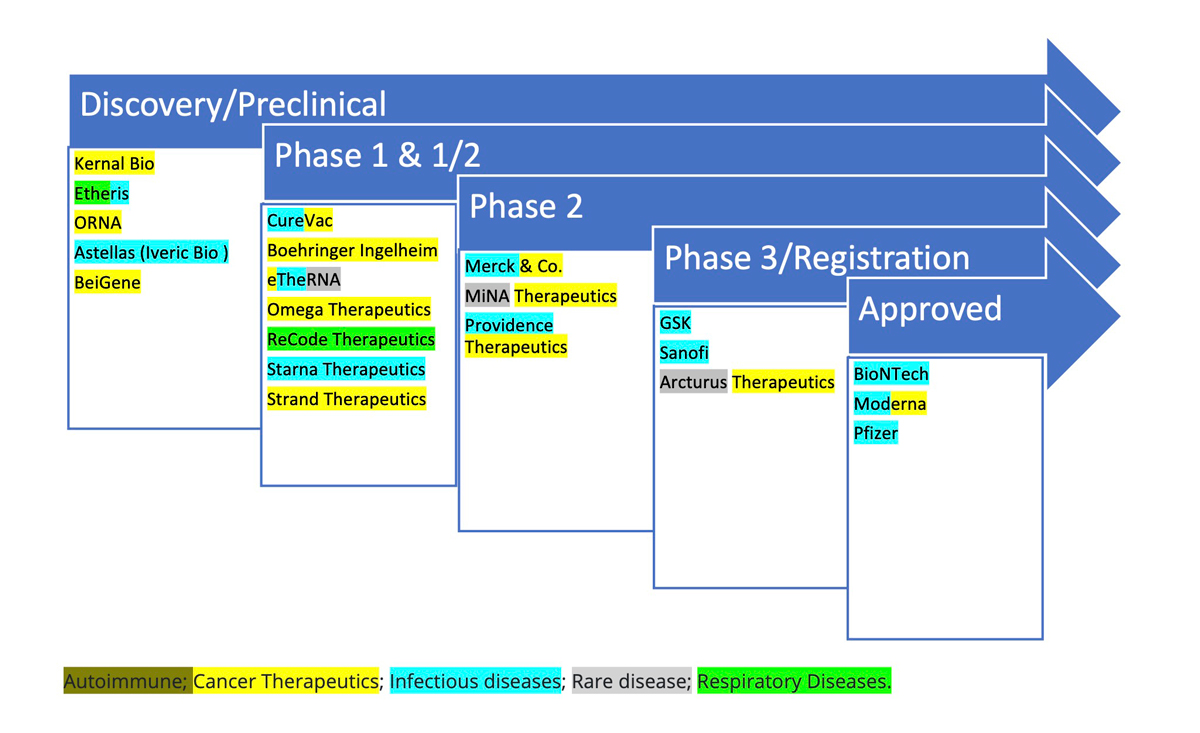
Source: modified from Pannu, 2024.
Vaccines for infectious diseases
The success of mRNA-based COVID-19 vaccines has heralded a new era in vaccinology. More than 90 clinical trials are now underway to develop mRNA vaccines for a broad range of infectious diseases, such as cytomegalovirus (CMV), human immunodeficiency virus (HIV), influenza, rabies, respiratory syncytial virus (RSV), severe acute respiratory syndrome (SARS), and Zika virus - administered as formulated or non-formulated mRNA injections using lipid nanoparticles (LNPs) and other non-viral delivery platforms.
mRNA vaccines offer clear advantages and disadvantages compared to traditional vaccines. According to Dr Erik Wiklund, CEO of Circio, Norway, “mRNA vaccines are fast to manufacture and manufacturers can quickly and precisely alter the antigen sequence to generate new versions. For RNA viruses that mutate frequently, like COVID-19 and influenza, manufacturers can quickly adjust mRNA vaccines. The caveat is, that mRNA is not particularly immunogenic compared to other vaccine formats, and experience from COVID-19 suggests that multiple doses are required for robust protection.”
Dr Wiklund added that mRNA vaccines require cold chain storage, so aren’t ideal for use in more difficult locations. He explained: “For more permanent pathogens, for example, polio or measles, live attenuated vaccines still appear to provide a broader and more durable immune response with a single dose. Therefore, mRNA vaccine design, formulation, and delivery will need to be further improved to compete in this area; and self-amplifying RNAs (saRNA) may become a preferred platform, due to stronger immunogenicity vs mRNA.”
Several pharma companies – Arcturus Therapeutics, Bayer, GSK, Moderna, Pfizer, and Sanofi - are all racing to develop mRNA influenza vaccines. GSK has teamed up with CureVac and currently has vaccine candidates for seasonal influenza and COVID-19 in Phase 2 and avian influenza in Phase 1 clinical development. Sanofi is developing monovalent MRT5407, MRT5410, and MRT5413, quadrivalent mRNA influenza vaccines (QIVs) in Phase 1/2. However, it has not all been plain sailing. Pfizer recently terminated the development of its Phase 2 respiratory vaccine combination PF-07960613, designed to protect against RSV and COVID-19.
GSK, Sanofi, and Moderna are also targeting RSV, the underlying cause of the common cold. Moderna’s mRESVIA (mRNA-1345 LNP) encodes RSV membrane-anchored prefusion F (preF) glycoprotein and received FDA approval in May to treat RSV in adults aged 60 and older. Sanofi’s RSV/hMPV mRNA LNP is in Phase 1 development to treat RSV or human metapneumovirus (hMPV) in older adults. And China-based Starna Therapeutics has utilised its proprietary STAR (selective targeted RNA delivery) LNP technology platform to develop its mRNA vaccine STR-V003, currently in Phase 1/2 study for RSV.
Leading academic institutes are also working on developing new universal influenza vaccines. In the US, the National Institutes of Health (NIH) is running a Phase 1 trial with a 'mosaic' vaccine, based on a virus-like particle (VLP) that displays hemagglutinin (HA) antigens. The National Institute of Allergy and Infectious Diseases (NIAID) Vaccine Research Center (VRC) is testing H1ssF-3928 mRNA-LNP, an mRNA-based vaccine coding for the tail portion of the HA antigen. Meanwhile, researchers at Oregon Health & Science University (OHSU) have developed a CMV vector that could confer lifetime immunity against evolving viruses, including the highly pathogenic avian influenza strain H5N1, which has the potential to cause a new pandemic.
Smart mRNA-based cancer therapeutics
Beyond vaccines, pharma is looking to develop mRNA vaccines to treat diseases requiring protein expression, such as cancer. Cancer has proved to be a graveyard for vaccine developers, however, mRNA vaccines could offer a new approach to induce both innate and adaptive immunity. Importantly, they can be personalised to encode specific antigens, do not run the risk of insertional mutagenesis, and lend themselves to cost-effective, large-scale production.
Several mRNA cancer vaccines have entered clinical development targeting haematological and solid cancers (e.g., colorectal, gastric, glioblastoma, melanoma, non-small cell lung cancer, oesophageal, ovarian, pancreatic, and prostate). Here, the synthetic mRNA encoding tumour-associated or tumour-specific antigens can be delivered through autologous dendritic cells engineered with mRNA ex vivo or through formulated or non-formulated mRNA injections.
Dr Wiklund noted that: “mRNA is particularly relevant to individualised cancer vaccines because it is a highly adaptable molecular platform. We have seen encouraging data with Moderna’s personalised melanoma vaccine and BioNTech is due to present data on its mRNA-shared antigen melanoma vaccine at ESMO. At Circio, we are developing a protein-based polyvalent KRAS neoantigen vaccine that can induce robust KRAS-specific T-cell responses in the clinic as an off-the-shelf therapeutic cancer vaccine.”
Although therapeutic mRNA-based cancer vaccines have yet to be approved, encouraging results have been reported from early clinical trials either as a monotherapy or in combination with checkpoint inhibitors. In July 2024, BioNTech reported positive results from a phase 2 trial with BNT111 in combination with Regeneron’s checkpoint inhibitor Libtayo (cemiplimab) in melanoma patients and have also reported positive Phase 1 results with BNT122, lipoplex formulation in pancreatic cancer patients. In April 2023, Merck & Co (known as MSD outside of the US and Canada) and Moderna announced they had received PRIME scheme designation for LNP-delivered mRNA-4157/V940 following positive results in combination with Keytruda (pembrolizumab) in the Phase 2b KEYNOTE-942 trial in melanoma patients. And late last year, the companies initiated a phase 3 INTerpath-002 evaluating mRNA vaccine mRNA-4157 (also known as V940) in combination with Keytruda in non-small cell lung cancer (NSCLC).
Meanwhile, MIT spin-out company Strand Therapeutics has created saRNAs that can amplify protein expression and eliminate mRNA off-target expression. Strand recently completed a $97 million series A financing round, the funds of which have been used to move five saRNAs into Phase 1 development in solid tumours and haematological cancers. Strand is also looking to develop circular mRNAs that can be used to express full-length proteins for weeks at a time in haematological malignancies. CureVac has also been busy developing SAMs to target hard-to-treat glioblastomas and initiated a Phase 1 study with CV09050101 mRNA vaccine in June 2023; the study is due to be completed in March 2026. Companies still face many challenges in the optimisation of cancer vaccines, though: only last month GSK announced it had discontinued its Phase 2 HPV vaccine GSK4106647, for example, stating that it did not have best-in-class potential.
Future development of mRNA vaccines and therapeutics
The mRNA vaccine and therapeutics field is wide open for development and there is a large body of clinical evidence to support their use beyond the prevention of infectious diseases. To date, only anti-COVID-19 mRNA vaccines have received regulatory approval, and further work is needed to optimise antigen production to ensure adequate adjuvant effects in cancer, as well as autoimmune and rare diseases.
Despite these challenges, mRNA technology can be used to express large and complex proteins that are difficult or impossible to generate with current expression systems and mRNA constructs have also been used to express potent monoclonal antibodies for immunoprophylaxis. Further advances in mRNA biology, delivery, and manufacturing will pave the way for the development of a raft of novel mRNA-based vaccines and therapeutics in the near future.
Dr Wiklund is confident that cancer vaccines will become an integral part of the future therapeutic armamentarium, and that mRNA will likely dominate the personalised space due to its adaptability.
“However, we expect that linear mRNA will be superseded by circular mRNA because of its improved expression durability, longer shelf-life, and can be stored at higher temperatures,” explained Dr Wiklund. “In addition, circular mRNA vaccines will become significantly cheaper to produce, with up to 90% lower manufacturing cost, as they circumvent the need to use modified nucleotide analogues, and no 5´capping is required.”
“In addition, advances in delivery technologies over current gold-standard LNP approaches to improve immunogenicity and reduce dosing requirements will enable broader use of RNA vaccines in the future and likely replace older vaccine platforms,” he predicted.












