Case Study: MHRA

Following MHRA approval of a clinical trial, a study on Nuwiq, a recombinant FVIII therapy for the treatment of haemophilia A, can proceed.
Background
Haemophilia A is an inherited bleeding disorder caused by a lack of factor VIII, which causes blood clotting problems, such as bleeding in the joints, muscles, or internal organs.
 Patients with haemophilia A are at an increased risk of surgical complications, such as the poor healing of wounds, infection, and internal bleeding. Treatments are in development to reduce this risk and are progressing through clinical trials.
Patients with haemophilia A are at an increased risk of surgical complications, such as the poor healing of wounds, infection, and internal bleeding. Treatments are in development to reduce this risk and are progressing through clinical trials.
One such study has been submitted through the MHRA’s New Notification Scheme for low-risk phase 3 and 4 trials – those trials that compare new treatments with the best currently available treatment (phase 3) and after a drug has been licensed (phase 4).
Nuwiq phase 4 trial
Octapharma AG is a pharmaceutical company that develops and produces human proteins from human plasma and human cell lines.
The company submitted an application to the MHRA for a phase 4 trial to evaluate the efficacy of Nuwiq, a fourth-generation recombinant FVIII produced in a human cell line, in preventing bleeding in patients with severe haemophilia A who are undergoing major surgery while receiving emicizumab prophylaxis.
Nuwiq contains the active substance simoctocog alfa (human coagulation factor VIII), which helps the blood to clot. Nuwiq is used to correct the factor VIII deficiency by replacing the missing factor VIII, which provides temporary control of the bleeding disorder. The medicine is made by cells into which a gene (DNA) has been introduced, enabling these cells to reproduce it.
A new, risk-proportionate process
The company applied for its phase 4 trial under the MHRA’s new Notification Scheme for lower-risk clinical trials.
 Introduced in October 2023, the new scheme aims to reduce the time taken by the MHRA to approve the lowest-risk clinical trials by more than 50%.
Introduced in October 2023, the new scheme aims to reduce the time taken by the MHRA to approve the lowest-risk clinical trials by more than 50%.
The introduction of the new Notification Scheme followed the agency’s clinical trials consultation, which was endorsed by 74% of respondents. It forms a significant part of the MHRA’s overhaul of clinical trials regulation.
About 20% of UK initial clinical trial applications are expected to be eligible for the scheme. It is underpinned by a risk proportionate approach that relies on the sponsor demonstrating that the trial meets certain key criteria.
To access the scheme, sponsors register their interest directly with the MHRA. Guidance is then sent to the sponsor, detailing the information that is needed in a cover letter and notification scheme criteria form. This information is then submitted in the usual way to the Integrated Research Application System (IRAS) – the single system for applying for permissions and approvals in the UK.
Once received by the MHRA, the information is reviewed, and the trial is either accepted or objected to; the sponsor is updated within 14 days of the application's effective date. If the trial is objected, the application progresses through the full combined review CTA assessment route, which would include the co-assessment of the application by a Research Ethics Committee, as set out in the diagram below.
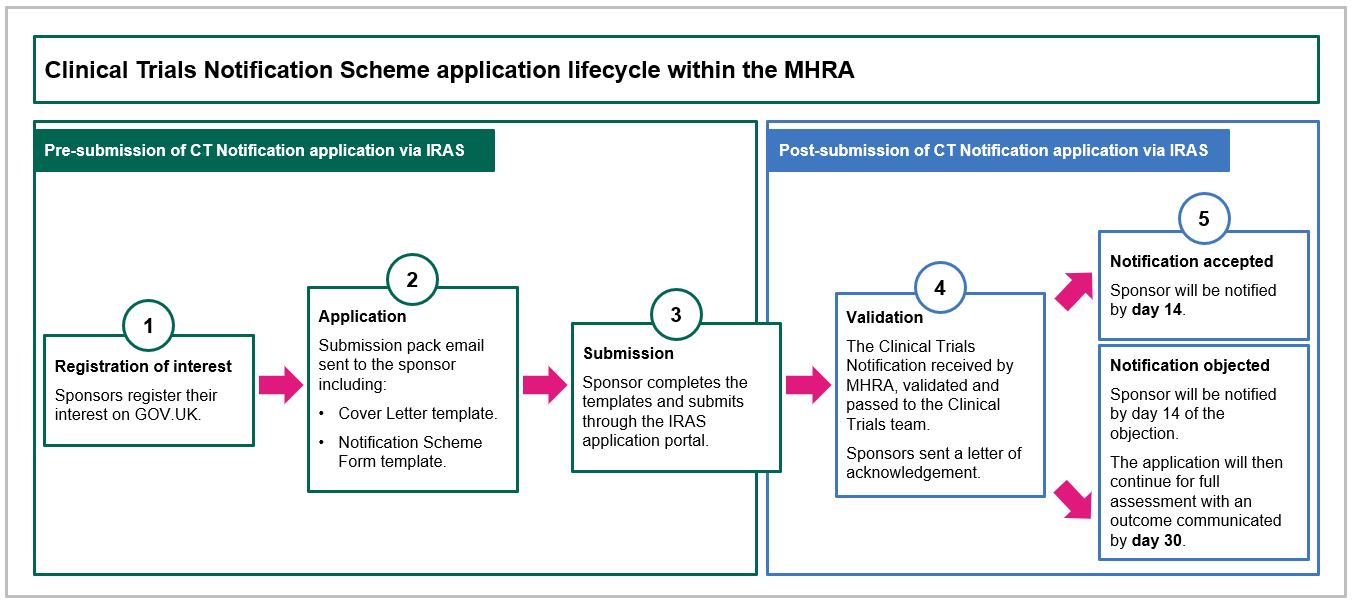
Full details of the new scheme are set out in the MHRA’s online guidance on clinical trials for medicines here.
Taking advantage of shorter timelines
As the trial submitted by Octapharma met the criteria for low-risk phase 4 trials, it was eligible for the new Notification Scheme. After being reviewed, the MHRA was able to notify Octapharma that the trial was acceptable under the notification scheme, by day 14.
“This case study demonstrates the effective operation of the MHRA’s new, streamlined clinical trials system, in reducing the time taken to get the lowest-risk clinical trials up-and-running without undermining patient safety.
“Clinical trials regulation should be flexible and risk-proportionate so that the regulatory requirements are geared to the risk that a trial presents.
“This will help give UK patients quicker access to the potentially life-saving medicines being studied.”
- Andrea Manfrin, the MHRA’s Deputy Director, Clinical Investigations and Trials.
The Notification Scheme is open for applications, and the agency’s message to all clinical trial sponsors is to use the new scheme for all eligible trials. Further information about the process, which sets out the criteria for eligible trials, is available from the MHRA here.
About the MHRA

An executive agency of the Department of Health and Social Care (DHSC), the Medicines and Healthcare products Regulatory Agency (MHRA) is responsible for regulating all medicines and medical devices in the UK by ensuring they work and are acceptably safe. All the agency’s work is underpinned by robust and fact-based judgements to ensure that the benefits justify any risks.












