AI-powered real-world evidence generation for biotechs

The biopharma industry faces a relentless demand for evidence of real-world effectiveness (RWE), safety, and value throughout the product lifecycle to complement the clinical trial-based benefit: risk profile from the pre-approval development programme.
A conventional Big Pharma approach would be to fund and develop an internal real-world data (RWD) capability comprising of research and programming staff, data scientists, an IT infrastructure to store and process large volumes of RWD, hefty fees to in-license data, and external vendors with access to specialised data, methods, expertise, or simply the ability to offer incremental capacity during busy periods.
But this option is not viable for the small biotech from the perspective of time or resources. Instead, these companies can learn from approaches pioneered by the technology industry.
Process thinking
- Prevailing mindset: The common process unfolds methodically, progressing through stages like hypothesis generation, feasibility assessment, and protocol development to analytics, study report generation, and peer-reviewed publication.
- Insight: The tech industry's ethos inherently embodies a process-oriented approach, characterised by a continuous cycle of investing in specific tasks and diligently striving to generalise and extend their product’s capabilities over time.
- Implication: This approach prioritises reusable RWD components, enhancing study efficiency and preserving knowledge. Even if initial goals are not met, these components can be applied to future studies, ensuring a more cost-effective and resilient research process. This methodology not only safeguards against knowledge loss from staff turnover, but also fosters continuous organisational learning and development within an RWD framework.
Continuous iteration
- Prevailing mindset: Due to the strict regulations the highest methodologic rigour in evidence generation is the norm.
- Insight: Entrepreneur Eric Ries introduced the 'lean start-up' in his book of the same name, where he argued that, in environments characterised by a high degree of uncertainty, the quickest path to success involves maximising the velocity of the “Build-Test-Learn” cycle.1 A higher tolerance for imperfection leads to faster initial results, proof of value, and broader stakeholder buy-in. Additionally, the technology industry places a higher value on early results, rather than waiting for a finalised product, as they use fast initial results to shape the future direction of their products.
- Implication: Clearly there is a need for balance. RWD must adhere to accepted research standards, however, there are certain project components that operate in 'safe spaces' where shorter iteration/review cycles of deliverables are beneficial, such as protocol development, data analysis, or report writing.
The role of technology
- Prevailing mindset: Those championing disruption to existing practices may encounter resistance due to concerns about data quality (i.e., the need to invest in technology to validate and enhance data quality). As computationally intensive applications, such as natural language processing (NLP) become more prevalent, many believe this skill should be internalised, adding further to resource demands on the ‘build’ model.
- Insight: Cloud providers like AWS, Azure, and Google offer Infrastructure as a Service (IaaS), where hardware maintenance is handled by the provider, and pharmaceutical companies are billed on a per-use basis. This means that resource-constrained biotechs do not need to construct and manage their own infrastructure.
- Implication: Enhanced access to physician notes and machine learning (ML) technology improves RWD quality,2 allowing clinical experts to make more informed assessments. This investment in technology boosts data reliability, builds trust, and enables more accurate effect estimates in studies. The advancement in causal ML further strengthens RWE studies,3 increasing their robustness and acceptance, and may support broader applications like label expansions.
Cross-functional teams
- Prevailing mindset: Too often, the classic approach for project management has been to work sequentially, moving tasks between different subject matter or functional specialists – for example, Medical Affairs to Health Economics and Outcomes Research (HEOR) RWE lead to a RWD statistician supported by RWD IT colleagues to scientific communications. This approach led to slowness and communication breakdowns, elevating the risk of delay and misalignment.
- Insight: It is paramount that a cross-functional team, with effective communication and collaboration, steers the projects. Technology companies’ principles of emphasising clear communication and the use of collaboration tools can be applied to biopharma to improve teamwork and efficiency. Also, cross-functional teams in fast-paced technology organisations must be adaptable and open to change to reflect the flexibility expected in today's dynamic business environment.
- Implication: Representation from the scientific side, as well as from the commercial side, is an obvious need. Leadership and accountability of the project could reside in any function, but we believe that the execution responsibility must be with a person with a dual background in computing and life sciences, especially in the early days: “The Translator”.4 The rationale is based on the necessity to keep an inexperienced team on track with a new technology. A skilled translator will act as the glue across business, science, and IT, helping the leader convert the outputs from the analyses into sensible, usable components of an enhanced value story or narrative. This role serves as a vital intermediary between the business and technology experts.
Figure illustrates the evolution of internal capabilities and staffing to advance RWE
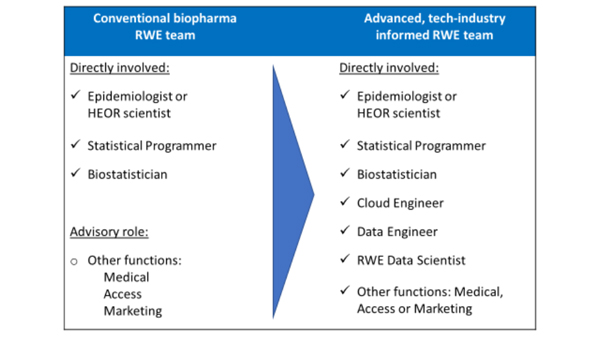
Change management
- Prevailing mindset: Biopharma organisations can still be siloed in the way they manage data. Clinical teams, including Regulatory Sciences and Biostatistics, are used in controlled environments and structured information, along with linear data collection and analyses to satisfy regulatory agencies, raising concerns when RWE generation departs from conventional practices (even though RWE is increasingly at regulatory quality grade).
- Insight: Many tech companies start with a clear vision and strategy for the change they seek, where they articulate the why, what, and how, ensuring alignment with business goals. Tech companies adopt agile methodologies for project management to allow for rapid iterations, continuous feedback, and adaptability to changing requirements. Finally, they implement pilot programmes to test changes on a smaller scale, gather feedback, and address issues before a full-scale rollout.
- Implication: Effective change management is crucial for adopting new methods like RWE, requiring stakeholder engagement and education to overcome resistance to new approaches. Involving end-users early, particularly in hypothesis design, and educating them on HEOR methods, enhances acceptance and trust. Advocacy across all levels ensures a smooth transition and realistic expectations for initial projects.
Given the relentless pressure facing biotech leaders on how best to allocate finite resources – time, people, and money - awareness of the potential ROI of an AI-informed capability to generate real-world evidence is business critical. Yet, as with any disruptive innovation, there will be challenges, champions, successes, and surprises.
In an ever-changing healthcare environment with patient, provider, and payer demands for evidence of real-world effectiveness, the conventional mindset of processes adopted wholesale from academia are ripe for change. We have had the good fortune to develop and apply a leaner, AI-informed RWE capability and encourage you to consider the potential it offers.
References
- The Lean Startup. Eric Ries. 2011.
- Real world evidence in cardiovascular medicine: ensuring data validity in electronic health record-based studies. https://academic.oup.com/jamia/article/26/11/1189/5548084
- Causal Machine Learning: A Survey and Open Problems. https://arxiv.org/pdf/2206.15475.pdf
- Generating real-world evidence at scale using advanced analytics. March 2022. https://www.mckinsey.com/industries/life-sciences/our-insights/generating-real-world-evidence-at-scale-using-advanced-analytics
About the authors
 Alexander John Büsser is head of product at Exploris Health. He is an expert in computational precision medicine, digital health, and real-world evidence, with 12 years’ experience across consulting, technology, and biotech sectors, including roles at IBM, Swisscom, and Idorsia Pharmaceuticals. Holding a postgraduate degree in Computer Science from École Polytechnique Fédérale de Lausanne, Büsser's expertise in machine learning has garnered him recognition through innovation awards and contributions to publications including Nature Medicine.
Alexander John Büsser is head of product at Exploris Health. He is an expert in computational precision medicine, digital health, and real-world evidence, with 12 years’ experience across consulting, technology, and biotech sectors, including roles at IBM, Swisscom, and Idorsia Pharmaceuticals. Holding a postgraduate degree in Computer Science from École Polytechnique Fédérale de Lausanne, Büsser's expertise in machine learning has garnered him recognition through innovation awards and contributions to publications including Nature Medicine.
 Christophe Segalini is a senior biopharma commercial executive with deep expertise across the business functional areas. He recently was senior VP of global value & access for a publicly traded start-up in Basel, Switzerland. Segalini has 25+ years of successful commercial-based experience, from the start-up environment through to blue-chip companies, across multiple countries and regions. Segalini holds a PharmD from the University of Lille, France and an MBA from the Kellogg School of Management, Northwestern University.
Christophe Segalini is a senior biopharma commercial executive with deep expertise across the business functional areas. He recently was senior VP of global value & access for a publicly traded start-up in Basel, Switzerland. Segalini has 25+ years of successful commercial-based experience, from the start-up environment through to blue-chip companies, across multiple countries and regions. Segalini holds a PharmD from the University of Lille, France and an MBA from the Kellogg School of Management, Northwestern University.
 Ross Maclean, MD, PhD, is executive VP and head of medical affairs at Precision Value & Health. Dr Maclean has over 30+ years of experience as a physician-scientist spanning medical practices in primary care and occupational medicine and health services research across diverse settings, including the UK National Health Service, a regional BlueCross BlueShield plan, the Veterans Health Administration, and a large academic medical centre. In his time in the biopharmaceutical industry, he has gained wide exposure to the R&D environment as a leader of the global health economics and outcomes research (HEOR) function for cardiovascular, metabolic, and immunology products. Dr Maclean has held executive-level leadership roles within a pharma setting as VP, HEOR for the US business and VP, diabetes and cardiovascular medical, both with BMS. He received his doctoral degrees from the University of Aberdeen in Scotland. In addition, he received a Master of Business Administration and a Master’s in Health Services and Public Health Research, also from the University of Aberdeen. He has contributed to more than 90 publications in peer-reviewed journals such as the American Journal of Managed Care, the Journal of Occupational & Environmental Medicine, Health Affairs, and the Journal of Medical Economics across a broad spectrum of topics, including telemedicine, healthcare quality, kidney transplantation, and rheumatoid arthritis.
Ross Maclean, MD, PhD, is executive VP and head of medical affairs at Precision Value & Health. Dr Maclean has over 30+ years of experience as a physician-scientist spanning medical practices in primary care and occupational medicine and health services research across diverse settings, including the UK National Health Service, a regional BlueCross BlueShield plan, the Veterans Health Administration, and a large academic medical centre. In his time in the biopharmaceutical industry, he has gained wide exposure to the R&D environment as a leader of the global health economics and outcomes research (HEOR) function for cardiovascular, metabolic, and immunology products. Dr Maclean has held executive-level leadership roles within a pharma setting as VP, HEOR for the US business and VP, diabetes and cardiovascular medical, both with BMS. He received his doctoral degrees from the University of Aberdeen in Scotland. In addition, he received a Master of Business Administration and a Master’s in Health Services and Public Health Research, also from the University of Aberdeen. He has contributed to more than 90 publications in peer-reviewed journals such as the American Journal of Managed Care, the Journal of Occupational & Environmental Medicine, Health Affairs, and the Journal of Medical Economics across a broad spectrum of topics, including telemedicine, healthcare quality, kidney transplantation, and rheumatoid arthritis.












