For neurological disease, solving measurement is the first and biggest hurdle to re-imagining treatment
Neurodegenerative diseases like Parkinson’s and Alzheimer’s affect millions of people worldwide, and their prevalence is only increasing, according to the World Health Organization. And while pharma companies are working hard to find treatments for these diseases, they have a major problem that must be addressed before that work can be done effectively: the lack of objective, quantitative measurement tools for assessing the progression – or even the presence or absence – of these conditions.
“Your primary endpoint is now down to a subjective measure in different trial centres, perhaps done by different people with different subjectivity,” says David van Zuydam, CEO of Head Therapeutics. “And now we're going to determine whether this drug is actually performing or not over a certain period of time, using this big subjective system? So you can only imagine things can go wrong in that space.”
Last month, I had the privilege to attend a private event held by pharma-focused startup accelerator PharmStars where 11 startups organised around the theme of “Innovations in Neurological Disease” presented their work. To one degree or another, almost all were focused on this measurability problem. Utilising new digital technology, it turns out there are a number of ways to introduce objective measurement into the world of neurodegenerative diseases.
[caption id="attachment_94696" align="alignnone" width="1261"] Image source: courtesy Head Diagnostics[/caption]
Image source: courtesy Head Diagnostics[/caption]
The scope of the problem
The lack of measurability in neurological diseases plagues pharma every step of the way when it comes to drug development. Just recruiting for a trial of a drug that’s meant to intervene at an early stage of Parkinson’s or Alzheimer’s is difficult, because patients are almost never diagnosed until they’re symptomatic.
“If you're a pharma company, your biggest headache is how expensive it is to actually put together one of these trials,” James Hamet, CEO of Vistim Labs, told pharmaphorum. “And the reason it's so expensive is because most of the patients who you recruit and who you care for and who you manage don't show any type of progress.”
Even when patients are found, there are a number of issues with tracking their disease progression well enough to assess the performance of a drug. As van Zuydam mentioned, current assessment methods are paper-based, slow, and subjective.
“There are very few of these specialists that exist out there,” Shaun Patel, CEO of REACT Neuro said, describing the process of getting diagnosed with a neurological disease. “And if you're able to find them, it takes six to nine months to get an appointment with them. And if you make it all the way to that appointment, they'll print out a big stack of papers. It’s a nine hour paper-based assessment. It's laughable, right? It really is. And this is just the current standard of care that we have today.”
Besides being unwieldy, the paper tests aren’t especially predictive. One reason for that is that they, along with things like the gait test, can only be administered in a single moment, and neurological diseases can develop inconsistently, causing the patient to have up and down days.
“There are a number of tests that can be done in office,” says Gerry Chile, general partner and managing director of the digital therapeutics division at Healthware Group. “But what you miss is the full myriad of symptoms that happen day after day over a period of time and how external factors and the medicine they are taking and when they are taking it might impact on those symptoms. Like either alleviating them or not working well enough and so not keeping them under control.”
Promising biomarkers
The startups innovating in this space are looking for new biomarkers that are objective, precise, quantitative, and longitudinal. And it turns out that there are a lot of innovative approaches to measurement.
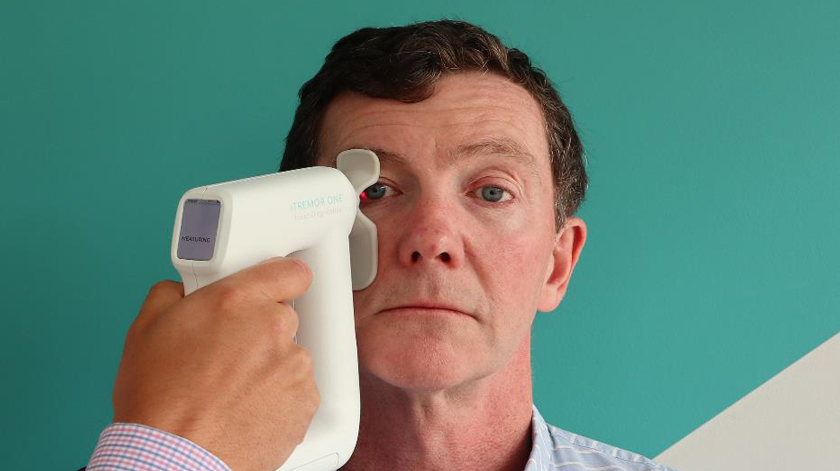
Head Diagnostics has a very straightforward, but very impressive test. A handheld device held up to the eye can detect ocular microtremor, an imperceptible twitch the frequency of which correlates reliably to the presence or absence of Parkinson’s Disease, among other neurological conditions.
Another startup, Accexible, has a high degree of accuracy using AI and vocal biomarkers, allowing them to detect and monitor mental health conditions over the phone or internet, which could be very helpful for trial recruitment.
“Usually in clinical trials for Alzheimer's, they do MRI, blood test, CSS, really aggressive different tests,” says Accexible CEO Carla Zaldua. “If you can do the 60-second test and you can see if somebody is going to be a good fit for the clinical trial, the potential our speech biomarkers have in order to help recruitment for a more diverse recruitment policy and also to lower the cost of clinical trial, it's amazing.”
Hamet’s Vistim Labs uses ECG to measure patients’ brains while they watch a specially-designed animation. They can compare the way the brain activates in response to that common stimulus with the behaviour of a healthy brain.
“We help pharma find people who are presymptomatic,” Hamet said. “We help them see how that person is changing month to month, not just on a symptom perspective. And what we aim to do and haven't done yet is a longitudinal study with one of these pharma partners so that we can predict where the patient will be in a couple of months. If we predict their symptoms, that's the best way to show that the intervention, by altering our prediction, is affecting the person's quality of life because those are symptoms avoided.”
REACT Neuro’s assessment tool immerses the patient in a VR environment and puts them through various exercises, all the while capturing data.
“Eye movements, voice, body, and head movements, all are captured in real time while you go through our REACT experiences,” Patel said. “We have a whole suite of these experiences that are designed to touch on all the aspects of neurological function from cognition, eye, head movement, balance, mood, and so on. Each administered via what we call a digital exam.”
From monitoring to treatment
Several of the startups at PharmStars used activity data from wearable devices to continuously monitor disease progression including Modus Health, Orbit Health, and HealthQb technologies.
One, Orbit Health, is taking things a step further, hoping to use wearable data not only to help pharma run trials, but also to have a direct value in Parkinson’s patients’ lives, determining whether they are in controlled, immobile, or hyperkinetic states and helping their care team to adjust their medication dosage in response.
“Every Parkinsons patient is different, so it's extremely individualistic and it's very unpredictable,” Orbit CEO Patty Lee says. “And so you can't really have like a one size fit all treatment regime for every patient. … We offer those currently missing insights to enable that personalisation of treatment to happen, because we are able to continuously and objectively monitor the patient's motor symptoms as well as treatment response.”
Soturi, a product co-developed by Healthware and Orion Pharma, was not at the PharmStars event but is working in a similar vein to the startups that were present. It offers an app and wearable combo that collects data from Parkinson’s patients to help increase understanding of the disease, while also providing tools that are helpful to the patient with things like medication reminders, physical exercises, and stress management techniques.
[caption id="attachment_94728" align="aligncenter" width="504"] Image source: Soturi[/caption]
Image source: Soturi[/caption]
“And ultimately, what will come out of this app is a recommendation to the treating physician that, hey, for this patient, here is what their symptomology was over time since the last appointment,” Chille said. “And based on our pharmacological model for (Parkinson’s drug) Levodopa, we recommend that the patient should have this dosage and this frequency with dispensive changes. So we're about a year away from this particular algorithm.”
The future of neurological care
For their first steps, these startups are targeting pharma, hoping to help them tackle the very real problems with running trials for these conditions. But they all have steps planned beyond that. Tools like Head Diagnostics’ device could some day place a screening for Parkinson’s and Alzheimer’s in every optometrist’s office along with tests for glaucoma and cataracts. REACT Neuro’s team hopes to be a home diagnostic device for mental health. And Orbit’s Lee hopes some day to create a closed-loop Parkinson’s therapy like the closed-loop solutions being developed for insulin management in diabetes.
If even a few of these startups’ visions are realised, the patient journey for neurodegenerative conditions could be set to change drastically.
As for PharmStars, the organization that brought these startups together and connected them to some of their first pharma partners, they are accepting applications for three more days for their next cohort, focused on “Innovations in Real-World Evidence.”
Feature image credit: courtesy Head Diagnostics












