Automating clinical trials: Why it’s essential for success

Many companies in the life sciences industry are slow to adopt new technologies. This is down to greater costs, greater operational burden, and uncertainty with meeting the complex regulatory requirements put in place. However, there has been a lot of advancement in new and emerging technologies in recent years. The FDA has recognized their benefits and is increasingly supportive of their use.
Running clinical trials costs a lot of money! Despite that, there’s no guarantee of success. Many clinical trials fail. And there are many reasons why. For example, it can be difficult to recruit patients or they may drop out during the trial. Badly designed forms can result in incomplete data being collected. There’s a huge amount of complex data to deal with. The list goes on.
The time it takes to get a product to the market is critical for companies. Each extra day amounts to greater costs and a loss of competitive advantage.
Automation can mean the difference between failure and success!
What is meant by automating clinical trials?
Automation is used to improve the accuracy of results in the clinical trial process from study setup through to submission. It removes manual processes such as creating forms and annotations by hand or entering data manually onto a spreadsheet. Results are accurate as human error is reduced.
It also results in a simplified, more streamlined process. The hard work is done for you automatically and often at the push of a button. So that’s less time and effort required to get more accurate results.
Automation also makes it easier to comply with the standards required by regulatory authorities.
5 benefits of automating clinical trials
5 main benefits are:
- Faster clinical trials from start-up to completion.
- Save time, resources, and costs.
- Better quality data means safer, more accurate clinical trials.
- Results can be compiled and analyzed faster.
- Better productivity throughout the clinical trial lifecycle.
These benefits result in a greater likelihood of successfully getting clinical products to the market on time and within budget.
Areas where automated processes make clinical trials more efficient
Case report form development
This refers to data that's gathered during clinical trials by way of case report forms (CRFs or eCRFs). The process of developing forms can be automated. Validation is built-in so that forms are completed correctly and regulatory requirements are met. Reporting tools allow for data analysis. Using software that automates form design results in better data quality, a faster data collection process, and saved costs during the lifetime of a clinical trial.
Clinical metadata management
Clinical metadata management refers to centralized systems that allow companies to store and manage their metadata assets. Such as forms, datasets, edit checks, and controlled terminologies for clinical studies. They have governance built-in and standardized metadata can be reused across many studies. The main benefits, aside from reuse, are regulatory compliance, increased data quality, and process efficiency.
Dataset conversion processes
During the various phases of a clinical trial, data must be submitted and resubmitted in the correct CDISC format to the FDA many times for review. Converting clinical trial data can be time consuming and expensive. By automating the dataset conversion process, there’s no need for complex programming. It’s also easier to meet regulatory requirements, and it takes less time to get clinical products to the market.
Why automation in clinical trials is essential
The costs and complexities of running clinical trials have never been greater. Without automation, getting high quality clinical products to the market today would be almost impossible. Huge amounts of data than ever is being collected and analyzed. There are more regulations in place. Standards are constantly being updated. Everything is so much more complex than it used to be. There’s no time like the present to highlight this with the COVID-19 pandemic at the forefront of medical research.
Fortunately, there are various technological solutions that automate processes for faster, effective, more efficient clinical trials.
The Formedix clinical trial automation platform and clinical metadata repository is one such system that incorporates automation into various areas of the end-to-end clinical trial process. It includes the automated processes discussed above and ultimately helps to get clinical products to the market faster.
How can Formedix help?
Our clinical metadata repository (MDR) lets you store and manage your metadata content. It lets you build studies quickly using built-in automated processes. Choose to build studies from scratch or upload existing organizational standards. Features include governance, impact analysis, and change management that you can see in the diagram below.
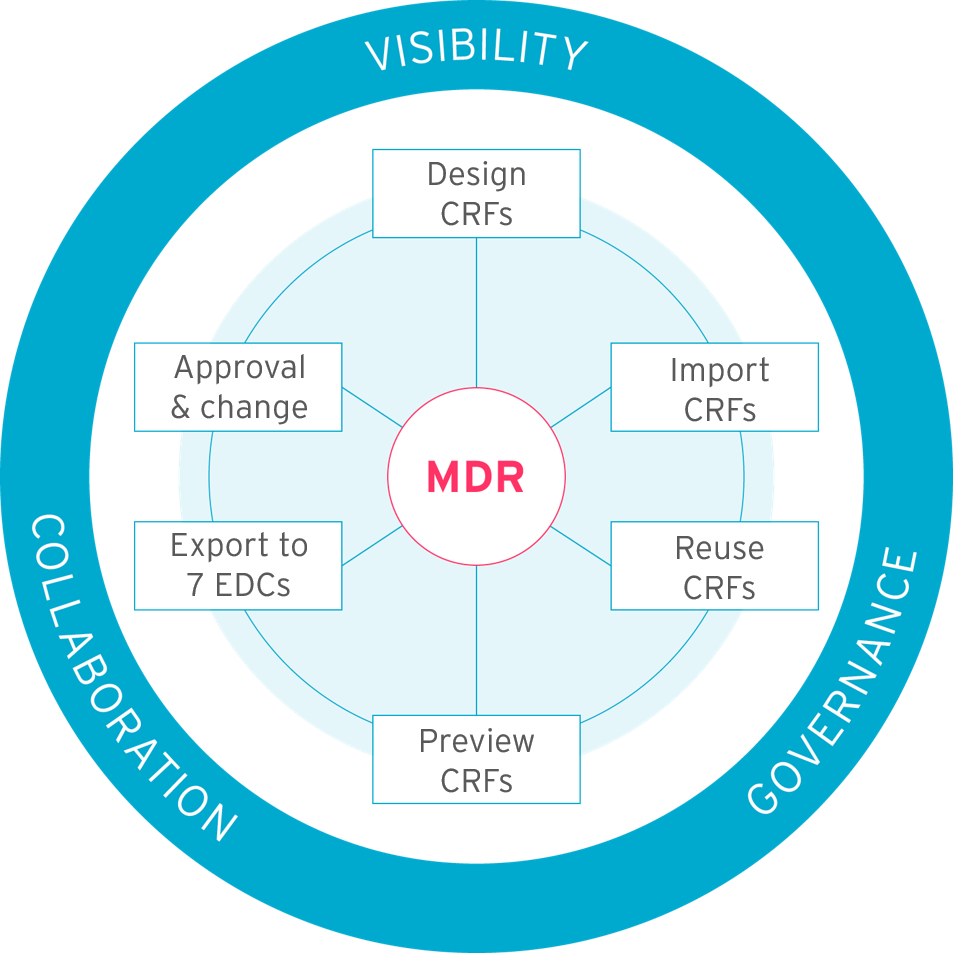
Quickly and easily do your eCRF designs in Formedix. You can create different metadata formats. Types of previews you can do include:
- CRF specifications.
- Edit check specifications.
- Visit structure specifications.
- Mapping specifications.
- SAS XPT clinical views.
- SAS v9 clinical views.
You can design your studies in a choice of 7 leading EDCs including Rave and InForm. You can see what your forms look like and how they’ll work in your chosen EDC as you design them. Then, with just 1 click, you can build your EDC database.
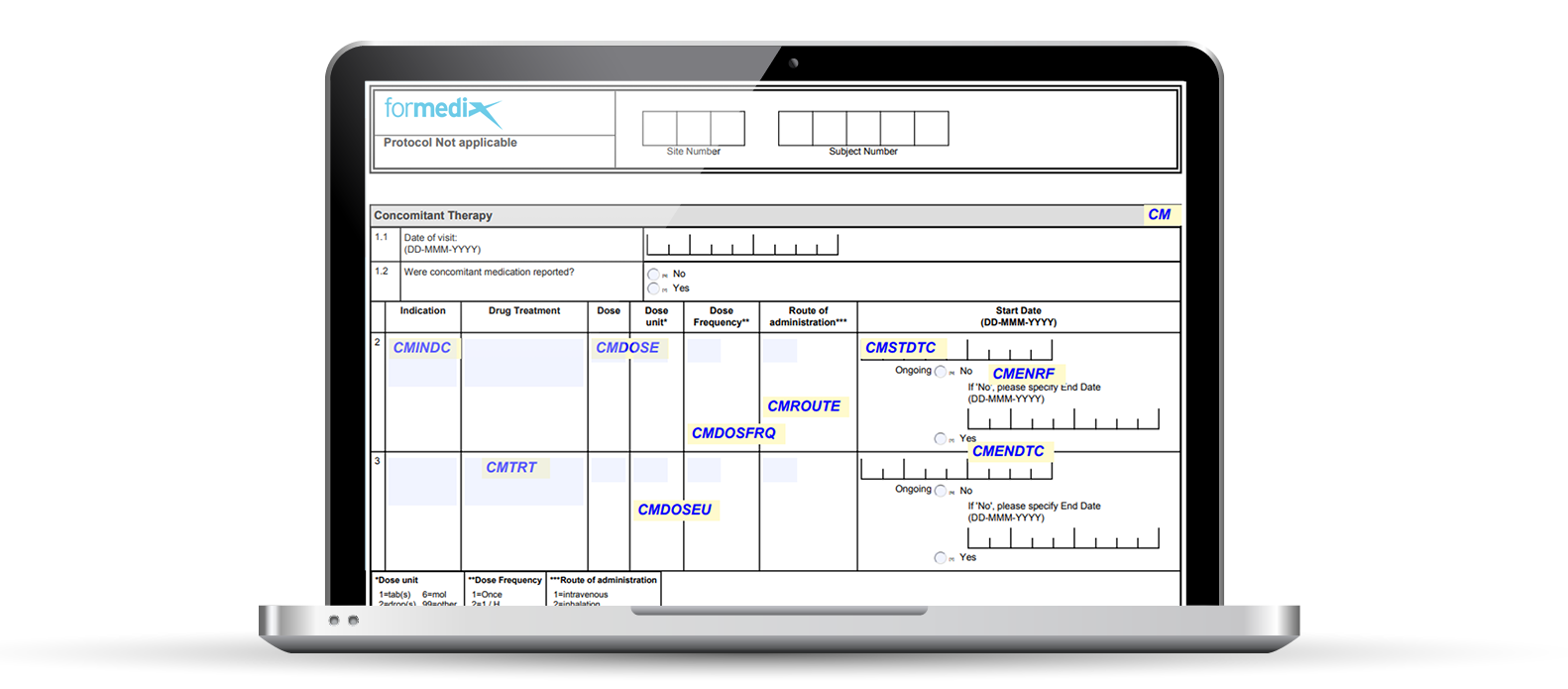
You can quickly and easily add annotations to your CRFs. Once your CRFs are standardized, you can just reuse your annotations. When your CRF design is complete, it’s 1 click to generate your submission ready annotated CRFs in PDF format.
The image below shows an annotated CRF in Formedix.
Formedix lets you generate SDTM datasets from source data in your EDC system. Simply define your mappings between source data to SDTM, then as soon as you start collecting data you can generate your SDTM datasets.
It’s 1 click to generate your SDTM define.xml after you’ve built your study and defined your datasets. And 1 click to generate your ADaM define.xml. You can also generate define.xml from legacy datasets and SAS XPT files.
Formedix supports all versions of CDISC and NCI standards. You can be safe in the knowledge that your study designs and datasets are regulatory compliant.












