Single-cell multi-omics and oncological potential
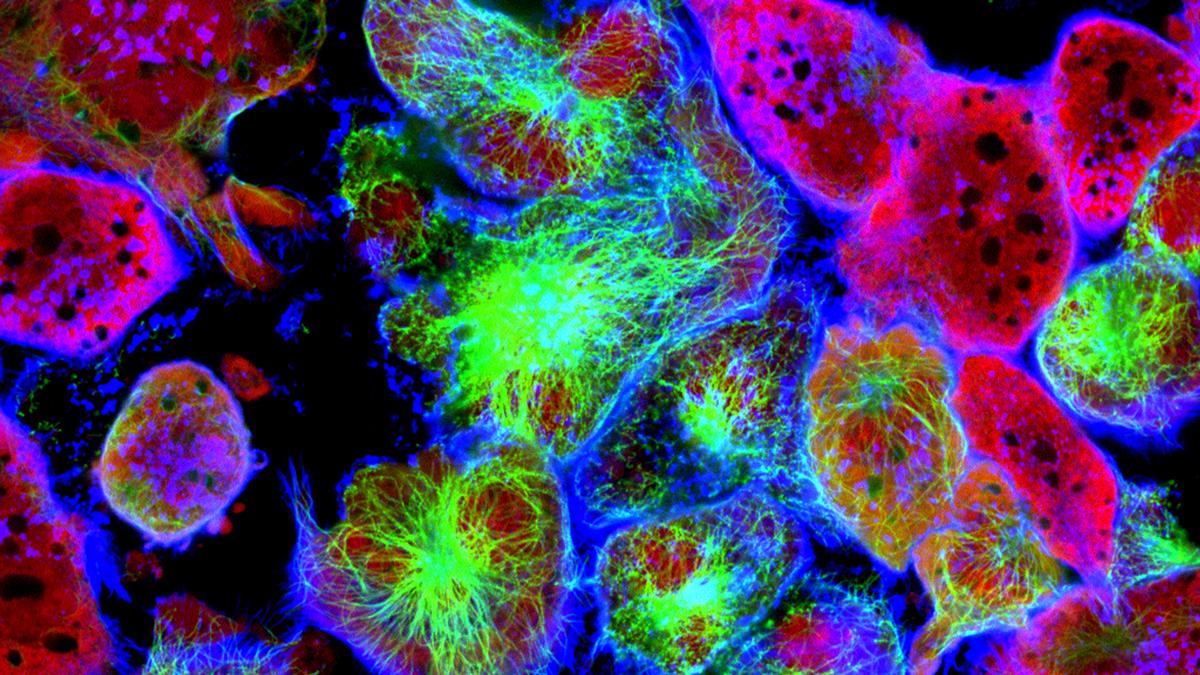
Just like people, no two cancer cells are alike. And now, scientists from the University of Surrey and SCIEX have come together and developed a method for studying the fatty content inside cells that could be key to the effectiveness of cancer treatments – fragmenting lipids and studying live cancer cells down to the single-cell level.
To find out more, pharmaphorum spoke with Professor Melanie Bailey, director of SEISMIC, theme leader for health and food technologies, EPSRC research fellow, and reader in analytical science at the University of Surrey, together with Rebekah Sayers, manager of global strategic marketing, small molecule omics, at SCIEX.
Cancer research and studying lipids cell by cell
In the new single-cell lipidomics study, ‘Researching cancer by studying lipids cell by cell’, led by the University of Surrey and published in the journal Analytical Chemistry, scientists sampled single live cancer cells and measured the fatty lipid compounds inside them. The results showed how the cells transformed in response to changes in their environment.
Lipids are an essential part of a cell’s biomolecular pool and play important roles in multiple and complex biological processes with functions ranging from the biophysical to energy storage and signalling. When it comes to cancer cells, which are highly heterogeneous, findings suggested that “the analysis of single cells and the characterisation of the heterogeneity could provide new treatment strategies” within the oncological space.
The research was undertaken by working with partners at GSK and UCL and developing new equipment with the Yokogawa Corporation in a process where individual pancreatic cancer cells were lifted from a glass culture dish using Yokogawa’s Single Cellome System SS2000, extracting single live cells using tiny tubes 10 µm across (approximately half the diameter of a thin human hair). Scientists stained the cells with fluorescent dye and monitored lipid droplets (stores of fatty molecules inside cells thought to play an important role in cancer) throughout the study, and SCIEX worked with them to develop a new method using a mass spectrometer to fragment the lipids in the cells and learn more about their composition and environmental responses.
Single- and sub-cellular ‘omics’: Radiobiology and precision therapeutics
A separate study published in the journal Genes (Basel) last year noted how we are now in an “omics era”, stating that “functional single-cell omics has improved our understanding of cancer heterogeneity,” as single-cell omics data can highlight cell-to-cell diversity and map the evolution of a cancer’s biological processes. Another paper published in the Biochemistry and Biophysics Reports journal in March this year noted that single-cell multi-omics technologies have “revolutionised” cancer research by permitting this examination of individual cells at a molecular level.
Single-cell multi-omics further enables identification of rare sub-populations of cells influential in tumour growth, metastasis, and resistance to therapy. It also offers valuable insights into immune responses triggered by various therapeutic interventions, such as immune checkpoint blockade, chemotherapy, and cell therapy. In short, single-cell multi-omics are a boon for progressing precision medicine.
Before speaking with pharmaphorum, Prof Melanie Bailey had previously commented on how the new research could help “better understand infection, immunity, and other phenomena as part of [a] new national facility for single and sub-cellular ‘omics’” called SEISMIC (of which Bailey is director), funded by the Biotechnology and Biological Sciences Research Council (BBSRC) and the Engineering and Physical Sciences Research Council (EPSRC). The team is also part of an International Atomic Energy Agency (IAEA) programme, exploring the effects of irradiation on cells.
As the IAEA website states: “Accelerator techniques play an important role in the study of radiation effects in biological cells and provide important information on DNA damage and response that can potentially be translated to clinical trials and improved health outcomes for radiotherapy. With recent developments in high-resolution ion beam and synchrotron-based X-ray microscopy imaging and sub-cellular targeting techniques, there is an opportunity to further develop, disseminate, and promote these techniques to better understand fundamental problems in radiobiology and cancer treatment.”
Prof Bailey’s facility sits with the UK National Ion Beam Centre (UKNIBC) and the EPSRC UKNIBC functions as a single point of access for the UK research community to undertake world-leading ion beam modification and analysis infrastructure and expertise. It is a collaborative delivery partnership between facilities at the University of Surrey’s Surrey Ion Beam Centre, the University of Huddersfield’s MIAMI & MEIS facilities, and the University of Manchester’s Dalton Cumbrian Facility. SEISMIC is the University of Surrey's third national research facility with the Ion Beam Centre and the Environmental Flow Laboratory. It is an open facility they are actively trying to give people access to.
“We've been comparing X-ray and proton beam irradiation, and we've also been comparing flash and conventional radiation,” explained Bailey. “Flash is a very high dose rate and radiation that is thought to selectively kill cancer cells spares rounding tissues, [but] we've just been first of all looking at bystander effects in cells.”
SCIEX and a triple partnership with GSK, UCL, and Yokogawa
In conversation with pharmaphorum about the oncological possibilities of her research, Bailey posited that her team is perhaps “a technology looking for a problem”, one which can be solved via collaboration.
“We have a long history of providing ion beams for customers for either analysis or materials modification, and one of the things that we've done for some time is […] study the impact of radiation on cells,” she explained. “Capillary sampling […] was just a capillary driven by a nano manipulator with the pressure injector [and] we found that we were able to pick up cells into these capillaries and then sprayed them into a mass spectrometer.”
Indeed, capillary sampling – as the Analytical Chemistry study notes in terms of its single-cell lipidomics – involves microscopy-based detection of adherent cells and sampling via glass capillaries under negative pressure, which has the advantage of preserving the spatial location of the cells, critical for understanding cell communication or “bystander effects”, as noted by Bailey. Nonetheless, “for untargeted lipidomics analysis of bulk samples, fragmentation-based identification of lipids via liquid chromatography-tandem mass spectrometry using data-dependent acquisition (DDA) has become the gold standard and has the advantage of automation.” The University of Surrey study, therefore, coupled DDA lipidomics to an automated cell selection system to provide a workflow for high-confidence lipid identification in single cells.
Having received £2.8 million in funding from the BBSRC and industry, the University of Surrey’s SEISMIC facility uses the advanced microscope manufactured by Yokogawa to enable single-cell and part-of-cell measurement to help fight cancer and infectious diseases by measuring the spatial position of biomarkers like proteins, metabolites, and lipids.
It was around the same time that SEISMIC was taking off that SCIEX’s ZenoTOF technology was invested in, a mass spectrometer. SEISMIC is currently working with the Pirbright Institute in respect of viruses and their impact on cells, as well as the University of Manchester on a study of allergens on cells. Indeed, as Bailey noted, there is a broad application to be had.
Furthering the exciting potential of single-cell analysis
SCIEX, which is now part of Danaher, aims to help customers solve the most impactful analytical challenges in quantitation and characterisation. For her part, SCIEX’ Rebekah Sayers – who holds a PhD in proteomics based mass spectrometry – explains that it has been the experience of working with single cells that has appealed to her academic background whilst working on behalf of the vendor in the arrangement with the University of Surrey, and that the cells themselves are rather “precious”.
Prof Bailey agreed with this appeal of single-cell work: “Understanding which lipids are implicated in like response to different stimuli, not just in cancer, but in any for any sort of biological problem - that was something that we were excited about.”
“You want to get the right amount of data out, then you have to balance your analytical methods, really, to get the sensitivity and the throughput, as well as to generate enough statistical power in these studies,” Sayers added. “Obviously, cells are very heterogeneous, so you need to have confidence in your analytical measurements that the variation you see is based on the biology, and not something that has come from the instrumentation.”
“Vendors like SCIEX really work with customers and try to listen closely to their research needs and build in different ways of looking at that for future developments,” she concluded.
Partnership, then – between academia and industry, and between traditional research methods and advanced technology – as well as an unfailing passion for the fundamental science are what look promising in furthering development of single-cell multi-omics research going forwards.












