Xeris’ severe low blood sugar pen gets FDA approval
After 14 years of operations Xeris Pharma has its first-ever product approval, claiming an FDA okay for a glucagon product as a rescue therapy for diabetics who develop dangerously low blood sugar levels.
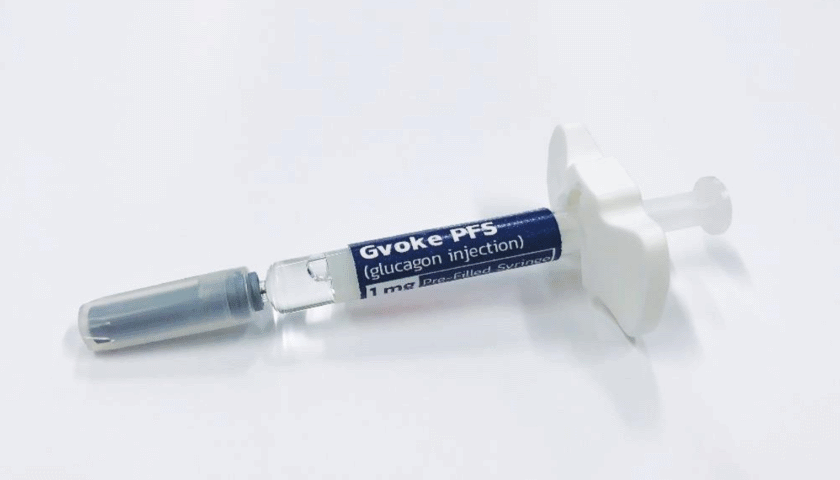
The product – which has been approved under the Gvoke brand name – is the first pre-mixed, ready-to-use glucagon to be licensed in the US for severely low blood sugar (hypoglycaemia), which can lead to coma or even death if left untreated. It has been approved in prefilled syringe and pen injector formats.
Glucagon has the opposite effect of insulin, which lowers blood sugar levels and can cause hypoglycaemia if given in too high a dose. Gvoke has been approved for use in diabetics aged 2 years and above.
Xeris chief executive Paul Edick said the company would press ahead with a launch of the prefilled syringe (Gvoke PFS) in the next four to six weeks, and debut the auto-injector version (Gvoke HypoPen) in early 2020.
The FDA’s decision on Gvoke was originally due in June, but was delayed by three months as a result of a major amendment to Xeris’ marketing application for the drug, specifically relating to chemistry, manufacturing and controls (CMC) data.
Gvoke is the second new glucagon product to be registered in the US in the last few weeks. Towards the end of July, the FDA approved Eli Lilly’s intranasal formulation of the drug, Baqsimi, which is the first non-injectable version of the drug.
Both are an improvement on older injectables that need to be prepared in a multi-step mixing and reconstitution process, so can be used to deliver the rescue therapy more easily, but for now the jury is out on which of the new drugs will prove most popular – and whether they can grow the market for hypoglycaemia rescue therapy.
Xeris says that having a ready-to-use formulation could increase the number of diabetics who have a hypoglycaemia rescue kit on hand in case of an emergency. At the moment that’s uncommon because diabetics and their carers are often reluctant to prepare current glucagon products so they generally need to be administered by a healthcare professional.
“Until now, many people may have been hesitant to use conventional glucagon kits because the complex preparation felt confusing and perhaps overwhelming,” commented Jeff Hitchcock, founder and president of medical charity Children with Diabetes.
“With Gvoke as a new glucagon option, we gain an easy to use and effective solution to a dangerous and stressful event,” he added.
Shares in Xeris were suspended yesterday morning in preparation for the FDA’s decision and tracked up after trading resumed, but closed the day down almost 11% on what appeared to be profit-taking – or perhaps concern about how well Gvoke will stack up against Baqsimi in the marketplace.
For now Xeris isn’t revealing its pricing specifics for the Gvoke products other than to say they will be pitched at the same level as current glucagon products. Baqsimi has a list price of around $280 per dose.












