FDA clears Lilly’s rescue med for severe hypoglycaemia in diabetics
The US regulator has approved the first non-injectable emergency medicine for diabetics who suffer potentially fatal low blood sugar levels.
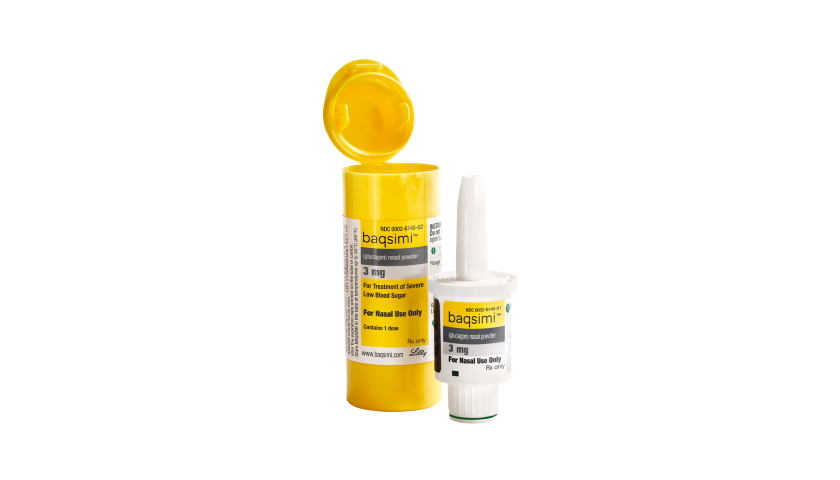
Eli Lilly’s intranasal formulation of glucagon – called Baqsimi – is the first alternative to injectable glucagon for severe hypoglycaemia, which typically occurs when people with diabetes take too much insulin.
Low blood sugar levels can lead to confusion, loss of consciousness and – if untreated – death, and injectable glucagon has been the go-to treatment for severe hypoglycaemic episodes for decades.
Lilly says the new product – which uses a delivery device developed by AptarGroup – is “compact, portable and ready to use”, which means it can be used more quickly than injectable glucagon products that need to be mixed in a several-step process. It delivers a single 3mg dose into the nose via a single-use dispenser, increasing blood sugar levels in the body by stimulating the liver to release stored glucose into the blood.
That time saving could make a life-or-death difference in an emergency situation, according to Janet Woodcock, the director of the FDA’s Centre for Drug Evaluation and Research (CDER).
“This new way to administer glucagon may simplify the process, which can be critical during an episode, especially since the patient may have lost consciousness or may be having a seizure,” she said.
“In those situations, we want the process to treat the suffering person to be as simple as possible.”
The efficacy and safety of Baqsimi was tested in two studies involving 83 and 70 adults with diabetes, which compared a single dose of Baqsimi to a single dose of glucagon injection. The intranasal drug “adequately increased blood sugar levels,” according to the FDA.
Similar results were also seen in a paediatric study of 48 patients over the age of four with type 1 diabetes. The FDA approval is for people with diabetes aged four years and above.
Lilly says it plans to launch the new product within a month and will price it at $280.80 for a one-dose pack and $561.60 for two doses – which is the same as injectable glucagon. It thinks eligible insured people with diabetes should pay as little as $25 for up to two devices if they use a savings card.
The US drugmaker acquired Baqsimi from Locemia Solutions four years ago, but didn’t disclose how much it paid for the rights.
It could face competition fairly soon from a pen-injector formulation of glucagon developed by Xeris, which is due for a verdict from the FDA in September after a three-month delay to its review caused by an agency request for more information.











