Sobi starts rolling FDA filing for chronic gout drug
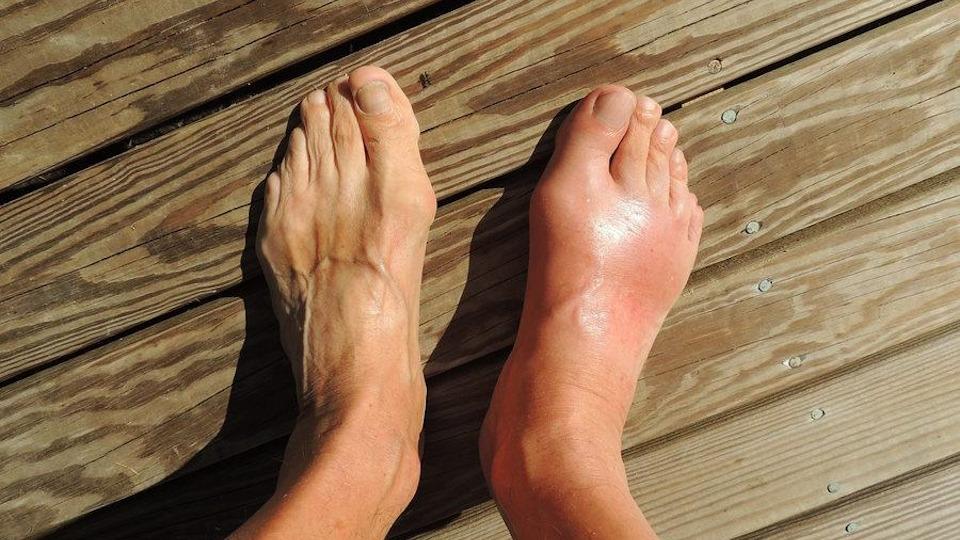
Sweden’s Sobi has kicked off a rolling biologics license application in the US for SEL-212, vying to become a new option for patients with refractory chronic gout that doesn’t respond to current treatments.
For patients with gout who cannot get relief using standard therapies, daily life can be a painful, debilitating experience. The disease is a painful form of inflammatory arthritis caused by elevated levels of uric acid in the blood resulting in crystals being deposited in and around joints, causing swelling, discolouration, and often excruciating pain.
Gout affects around one in 40 people in developed nations and is becoming more common as a result of a rising prevalence of risk factors such as high blood pressure and other chronic conditions like diabetes, obesity, and heart and kidney diseases.
Current first-line therapies like allopurinol and febuxostat can be very effective, but some patients – roughly 1% according to FDA estimates – don’t get the hoped-for benefit, while others are intolerant to the drugs.
Sobi’s filing is based on the DISSOLVE I and II pivotal studies, in which roughly half of the patients treated with SEL-212 at the highest dose tested hit a target uric acid levels in the blood of 1.5 mg/dL or lower for at least 80% of the sixth months after starting treatment. The current target of gout treatment is to reduce levels down to 6 mg/dL or less.
SEL-212 is based on a uricase enzyme (pegadricase) that breaks down uric acid in the blood combined with an immune tolerance component (ImmTOR) designed to prevent the formation of antibodies against the enzyme and prolong the efficacy of treatment.
It has been awarded a fast-track review by the FDA, which Sobi said “underscores the urgent need for new treatment options for patients with chronic refractory gout.”
If approved, it will compete with Amgen’s FDA-approved Krystexxa (pegloticase), another uricase-based therapy it acquired as part of its $28 billion takeover of Horizon Pharma, which closed last October. Amgen reported sales of $272 million for the drug after it took ownership in 2023 and $235 million in the first quarter of this year.
Sobi licensed SEL-212 from Selecta Biosciences – now called Cartesian Therapeutics – in June 2020 for $100 million upfront and milestone payments of up to $630 million. It is responsible for development, regulatory, and commercial activities in all markets outside of China.













