Senseonics, TypeZero to develop artificial pancreas
Medtech company Senseonics has signed a deal with diabetes management startup TypeZero to develop a new artificial pancreas option.
A wave of companies are looking to develop their own automated insulin delivery system for diabetics - technologies which could evolve into being a true 'artificial pancreas'.
These continuous glucose monitor (CGM) systems measure glucose levels around the clock, and automatically adjust insulin levels accordingly via an insulin pump.
One of the frontrunners in the field is Medtronic, which last year gained FDA approval for its MiniMed 670G,
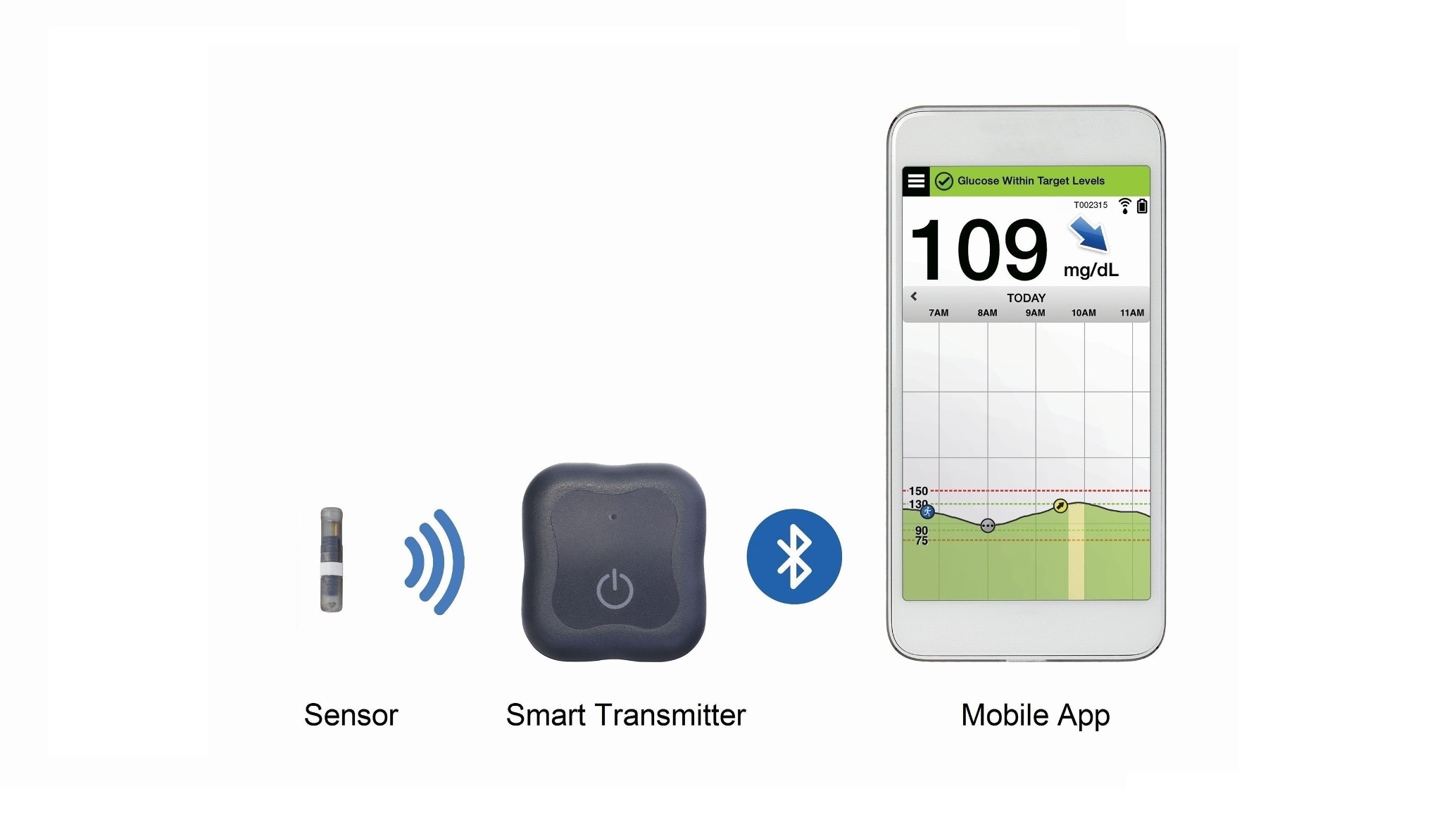
Senseonics already has a solution called the Eversense CGM. The system comprises an implantable sensor that lasts for up to 90 days, a removable smart transmitter and a mobile app.
The sensor reads glucose concentrations in the interstitial fluid under the skin and sends this data to the transmitter. This then calculates the current glucose value along with the direction it’s headed, how fast, and whether glucose values are expected to exceed pre-set low and high targets, and sends it to the app.
The new development deal will create a solution that feeds this data into inControl's artificial pancreas algorithms, which will then automatically adjust and regulate insulin delivery via the user's insulin pump.
The defining feature of inControl is its ability to predict high and low blood sugar levels, providing recommendations for optimal basal and bolus doses for insulin pen users. It can also administer correction boluses.
“We’re thrilled to partner with TypeZero with the goal of progressing the diabetes management field in providing solutions to help minimise the burden of diabetes for millions of people,” says Tim Goodnow, President and CEO of Senseonics. “The continuous long-term use of an accurate sensor such as Eversense combined with the clinically proven treatment algorithms of inControl has the promise of impacting both pump and pen users in a positive and meaningful way.”
The deal is the latest for TypeZero in its partnerships with CGM manufacturers. The company already has ongoing collaborations with medical device company Tandem Diabetes Care and Cellnovo. Both deals are integrating TypeZero's inControl platform into each company's respective devices: the Tandem t:slim insulin pump and Cellnovo's self-titled CGM system.
The inControl system is also being investigated by the US National Institutes of Health as part of the International Diabetes Closed Loop trial investigating the combination of inControl with Tandem and Dexcom insulin pumps.










