Respira Labs' wearable device listens for lung diseases
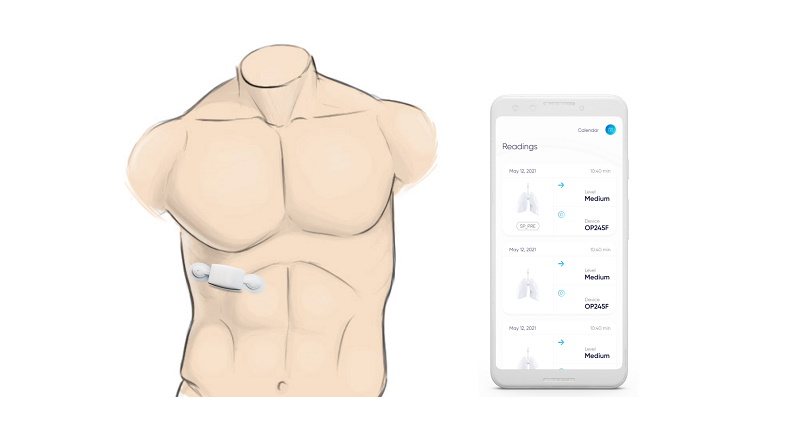
US company Respira Labs has developed a device worn on the chest that uses tiny speakers and microphones to project sound into the lungs and listen for signals that could indicate serious lung diseases.
The Sylvee wearable uses an artificial intelligence (AI) algorithm to interpret sounds that may indicate trapped air in the lungs, which can be a sign of ill health, using acoustic resonance – similar to the way an ultrasound machine creates visual images.
The California company says the device can be used to remotely monitor patients with COVID-19, chronic obstructive pulmonary disease, and asthma, and may serve as an early warning system for exacerbations that could prevent costly hospital visits. More than 100 million Americans are affected by COPD, COVID-19 and asthma.
"Well-established science shows that air trapping can be measured with more than 90% accuracy using low-frequency sound," said Dr Maria Artunduaga, Respira's founder and chief executive.
"There is a clear difference in the acoustic resonance spectra of COPD patients versus healthy controls," she added. Sylvee transmits the acoustic resonance data to a smartphone app, which interprets the findings.
Along with trapped air, the device also monitors lung function over time – using measures such as lung volume, capacity, rates of flow – to provide a comprehensive overview of a patient's condition, according to Respira, which says it has already secured Medicare reimbursement for the device.
At the moment, the standard of care relies on questionnaires and inaccurate pulse-oximeters, which according to the company miss around 50% of respiratory exacerbations leading to hospitalisations that can cost $19,000 to $50,000.
The hope is that using Sylvee could allow patients at risk to be identified and treated early, improving their chances of survival, although that still needs to be evaluated in clinical trials.
"The device facilitates early diagnosis and management of acute deterioration, which is what respiratory patients must avoid," said Dr Artanduaga.
"This is what happened with my grandmother," she continued. "She suffered from COPD and had a sudden exacerbation of her symptoms and tragically died. I left my medical career and devoted myself to devise the Sylvee – which is named after my grandmother – because of this terrible and common outcome."
The Sylvee device is currently in prototype with FDA clearance expected within the next 18 months.












