Neuralink gets okay for its first international trial
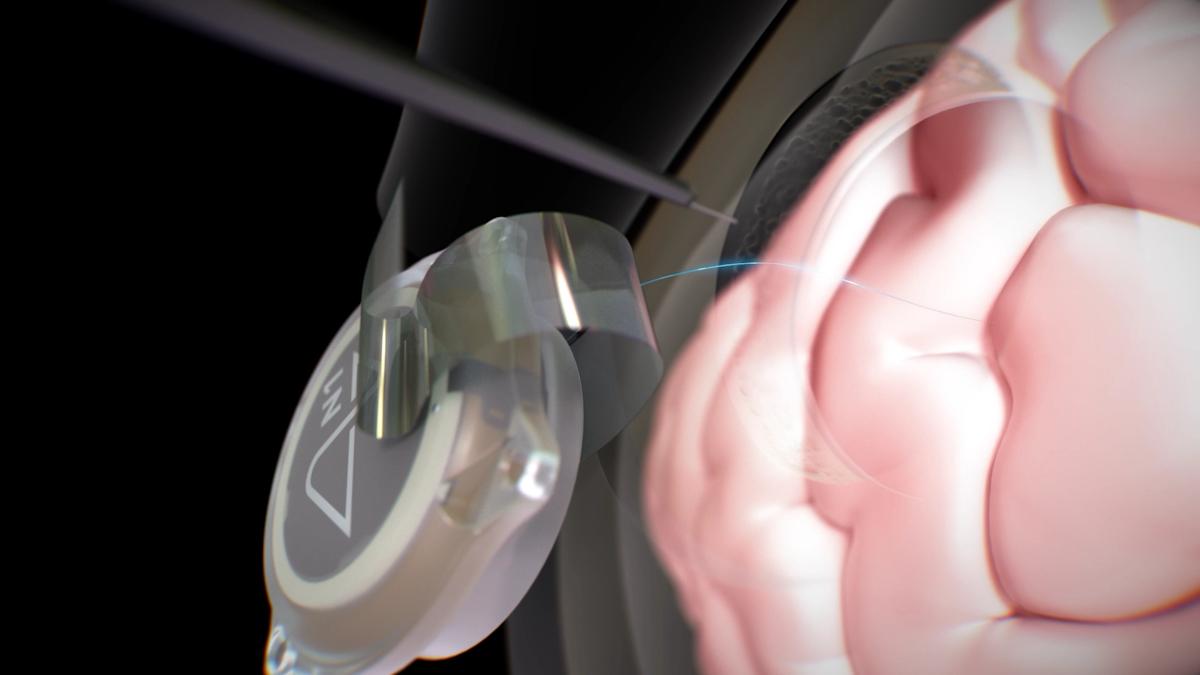
Elon Musk's brain-computer interface (BCI) company Neuralink has been given the green light to test its device in tetraplegic patients in Canada – the first international trial of the technology.
The CAN-PRIME study will run alongside Neuralink's recently initiated PRIME study in the US and will test the safety and functionality of its N1 implant in people who have difficulty moving their arms and legs to see if it can act as a hands-free interface between the brain and electronic devices, allowing them to be controlled with thoughts alone.
The "early feasibility" open-label study will also test the safety and efficacy of Neuralink's R1 robot that is used to implant the thread-like electrodes used in the BCI into the brain.
It aims to enrol half a dozen Canadian residents with severe tetraplegia (also known as quadriplegia) due to spinal cord injury or amyotrophic lateral sclerosis (ALS) and a life expectancy of at least 12 months.
The main objective is to gauge the safety of the implant and the surgical procedure to place it, but the study will also measure cognition – including attention, memory, language, and orientation – using the Montreal Cognitive Assessment (MoCA) scale, as well as anxiety and depression.
Follow-up will continue for up to six years, with four assessments of MoCA, anxiety, and depression carried out within the first 12 months.
Earlier this year, Neuralink revealed that the first patient to receive the N1 implant – called Noland – had shown promising signs of “neuron spike detection” suggesting the device had been implanted correctly, and he had been able to control a cursor on a computer screen.
Subsequently, there was some sign that the electrode threads had retracted, affecting the performance of the BCI, although the company has since said this has stabilised.
A second patient called Alex, who received the BCI in the summer, was able to start controlling a cursor with his mind within five minutes and was soon able to use the implant to play the first-person shooter game Counter-Strike. He has also started to learn how to use computer-aided design (CAD) software to design 3D objects, according to the company.
Neuralink says it is now working on adding functionality like multiple clicks and multiple simultaneous movement intents to deliver "full mouse and video game controller functionality."
It is also hoping to enable the device to interact with the physical world, allowing users to feed themselves and move more independently by controlling a robotic arm or their wheelchair.
Results from Can-PRIME are expected in 2027, according to the trial listing on the clinicaltrials.gov registry.












