Merck & Co's Keytruda gets new colorectal cancer use after month-long FDA review
It’s barely a month since Merck & Co unveiled trial results showing that cancer immunotherapy Keytruda (pembrolizumab) works in colorectal cancer patients with certain mutations, and the FDA has already added the new indication to its label after a speedy review.
The anti-PD-1 class drug has been approved in first-line treatment of patients with unresectable or metastatic microsatellite-high (MSI-High) or mismatch repair deficient (dMMR) colorectal cancer.
Approval is based on results from the phase 3 KEYNOTE-177 trial unveiled at the American Society of Clinical Oncology (ASCO) conference at the beginning of the month.
Results showed Keytruda significantly reduced the risk of disease progression or death by 40% compared with chemotherapy, the current standard of care.
The FDA granted approval less than a month following Merck & Co’s filing to extend Keytruda’s label, with the regulator using its Real Time Oncology Review pilot programme to speed up the process.
Results also showed a two-year progression-free survival rate of 48% with Keytruda compared to 19% with chemotherapy.
There was also an improved overall response rate with Keytruda of 43.8% compared with 33.1% with chemotherapy, and a complete response was observed in 11.1% and 3.9% of patients, respectively.
Partial responses were seen in 32.7% of patients in the Keytruda arm, and 29.2% of those in the chemotherapy arm and there were no surprises in terms of safety.
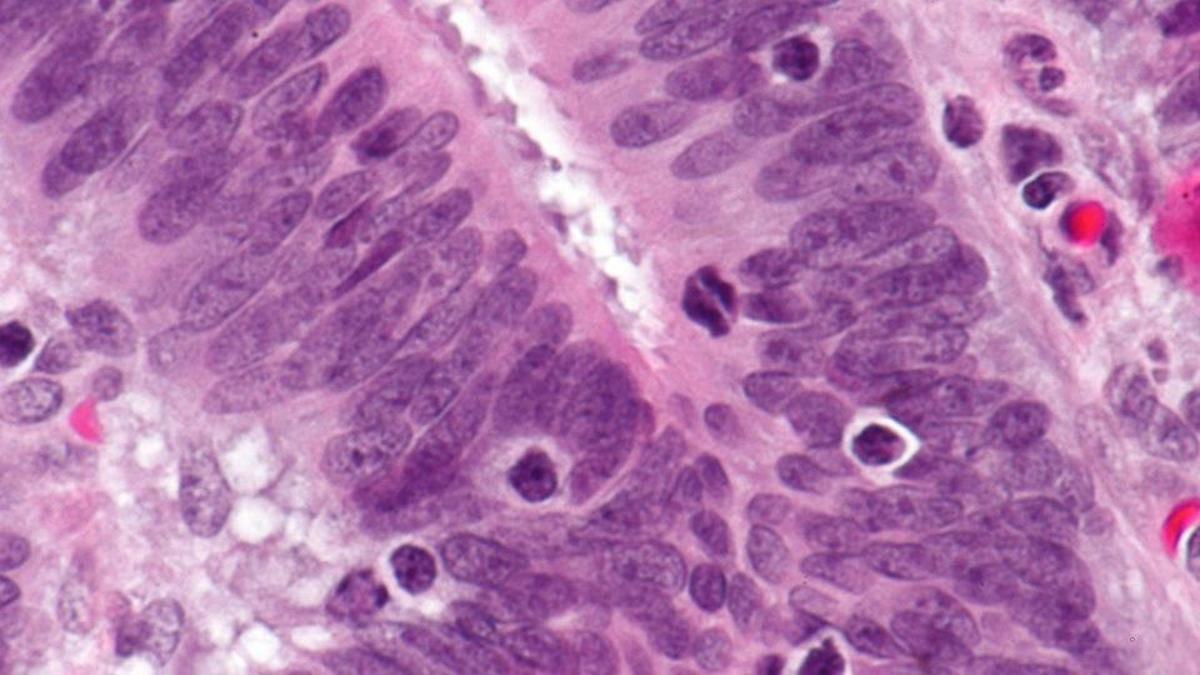
Microsatellite instability (MSI) is a change that occurs in the DNA of tumour cells where the number of repeats of microsatellites – short repeated sequences of DNA – is different from the number of repeats in the DNA in the body’s healthy tissues.
The cause of MSI may be a defect in the ability to repair mistakes when DNA is copied in the cell – referred to as a mismatch repair deficiency.
It is estimated that around 5-15% of colorectal cancer patients have tumours that score as either MSI-High or dMMR when testing is performed.
Keytruda was approved in 2017 in previously treated MSI-High or dMMR solid tumours, the first time a cancer therapy was licensed by the FDA for use on a biomarker, regardless of tumour type.
This review also was conducted under Project Orbis, an initiative of the FDA Oncology Center of Excellence that provides a framework for concurrent submission and review of cancer drugs among its international partners.