LEO says chronic hand eczema drug bests its only rival
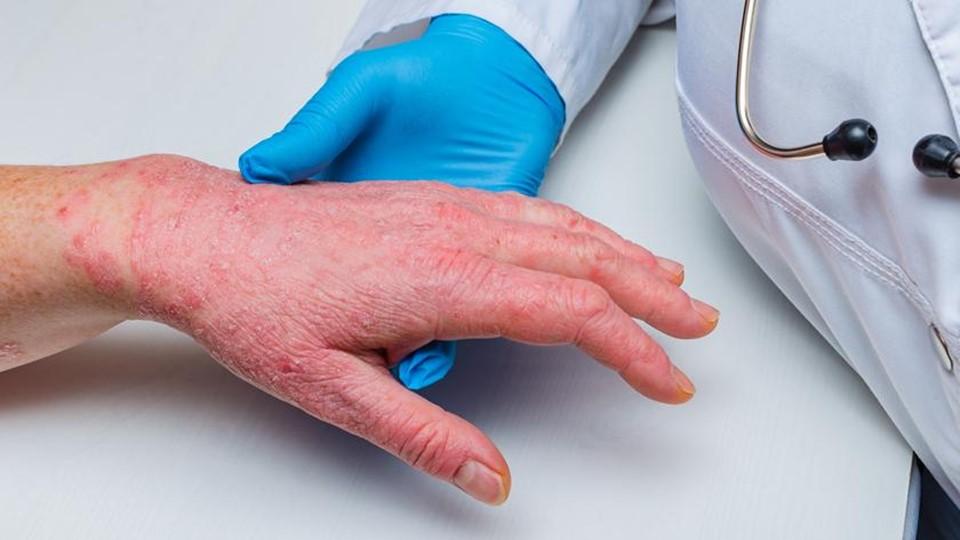
Dermatology specialist LEO Pharma has built the case for its first-in-class chronic hand eczema (CHE) drug delgocitinib, saying it outperformed the only approved drug therapy for the condition in a phase 3 trial.
LEO’s topical cream formulation of the pan-JAK inhibitor proved to be more effective than oral therapy alitretinoin in the DELTA FORCE study, which involved adults with moderate to severe CHE.
The 513-patient trial met its primary endpoint of a superior reduction in Hand Eczema Severity Index (HECSI) score from baseline to week 12 with twice-daily application of the cream compared to alitretinoin capsules.
For patients with moderate to severe CHE, a form of dermatitis, there’s no doubt symptoms like itch, cracking skin, and pain can severely affect quality of life. For these patients, standard therapies like emollients and topical corticosteroids are often ineffective, and too much use of steroids can thin the skin, eventually making symptoms worse.
“Our hands are integral to everything we do,” commented Alexander Egeberg, LEO’s head of global medical affairs.
“I have heard countless stories from patients about just how much this condition impacts their everyday lives socially, psychologically, and physically,” he added, noting that it often limits their ability to work.
In DELTA FORCE, delgocitinib also achieved secondary objectives, including a reduction in HECSI scores versus alitretinoin at 24 weeks and was better on physician-reported assessments of treatment success, as well as patient-reported quality-of-life scores.
Crucially, it was also better tolerated than alitretinoin, a drug that is known to cause side effects like nausea and vomiting and is restricted to carefully controlled use in patients unresponsive to topical corticosteroids because it carries a risk of causing birth defects if taken during pregnancy.
LEO has previously reported the results of two placebo-controlled phase 3 trials – DELTA 1 and 2 – which found that delgocitinib was better at alleviating itch and pain symptoms, in addition to providing significant improvements in HECSI scores. It has also been tested in a longer-term, open-label extension study (DELTA 3), showing safety and efficacy out to 36 weeks.
“With such a considerable unmet need for patients living with CHE, we are proud to have conducted this study, which we hope can drive forward a potential new treatment option with delgocitinib cream,” said Egeberg.
LEO filed for approval of delgocitinib in its first markets, including the EU, last year and is anticipating decisions before the end of this year. In 2020, the FDA granted breakthrough status for the drug as a CHE treatment.
Delgocitinib was licensed from Japan Tobacco in 2014, with LEO picking up exclusive rights to dermatology applications of the drug outside Japan. The company is also developing it for other uses, including alopecia, dry eye disease such as Sjogren’s syndrome, and some forms of psoriasis.












