Leo Pharma announces positive results for hand eczema study
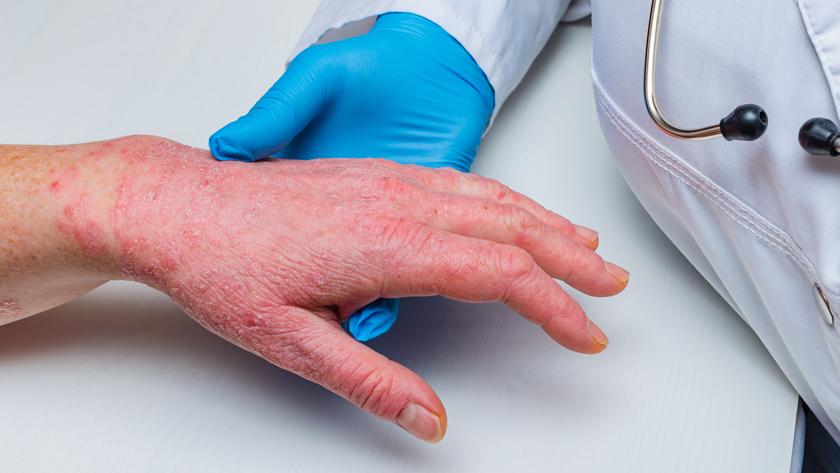
In good news for patients living with chronic hand eczema (CHE), Leo Pharma – a company working to advance medical dermatology treatments – has unveiled positive topline results for its delgocitinib cream treatment, an investigational topical pan-Janus kinase (JAK)-inhibitor, for the potential treatment of adults with moderate to severe chronic hand eczema.
Results from the phase 3 DELTA 2 study showed that delgocitinib cream met its primary endpoint of statistically significant improvement in CHE after 16 weeks of treatment compared to vehicle. This is the second of two phase 3 delgocitinib cream trials to achieve both primary and secondary endpoints, with outcomes from this latest study confirming findings from the DELTA 1.
During both double-blind, randomised, vehicle-controlled trials, participants received twice-daily applications of the cream to evaluate the efficacy of the treatment. The treatment was well tolerated, with all or most of the signs and symptoms of CHE cleared early in the process for the majority of patients given delgocitinib cream.
Secondary endpoints also included a reduction of itch and pain scores of ≥4 points measured by the Hand Eczema Symptom Diary from baseline to Week 16, as well as at least 75% improvement from baseline and at least 90% improvement from baseline on the hand eczema.
Outcomes from both trials have been an encouraging step forward for Jörg Möller, executive vice president, global research & development, LEO Pharma.
"It is incredibly exciting to see the level of consistency that our DELTA 2 results show in line with the positive DELTA 1 results announced late last year," he explained. "CHE is a condition that we know can have a hugely negative impact on patient quality of life, physical functioning, and ability to work. These results bring us one step closer towards establishing delgocitinib as a best-in-class innovative topical treatment for patients affected by this hard-to-treat disease."
Further analysis will now be conducted to determine the full potential of delgocitinib cream in the treatment of adults with moderate to severe CHE. Moreover, participants who completed 16 weeks of treatment in trials DELTA 1 or DELTA 2 were invited to roll over to the DELTA 3 extension trial to evaluate the long-term effects of the treatment.













