LEO closes on first approval for chronic hand eczema drug
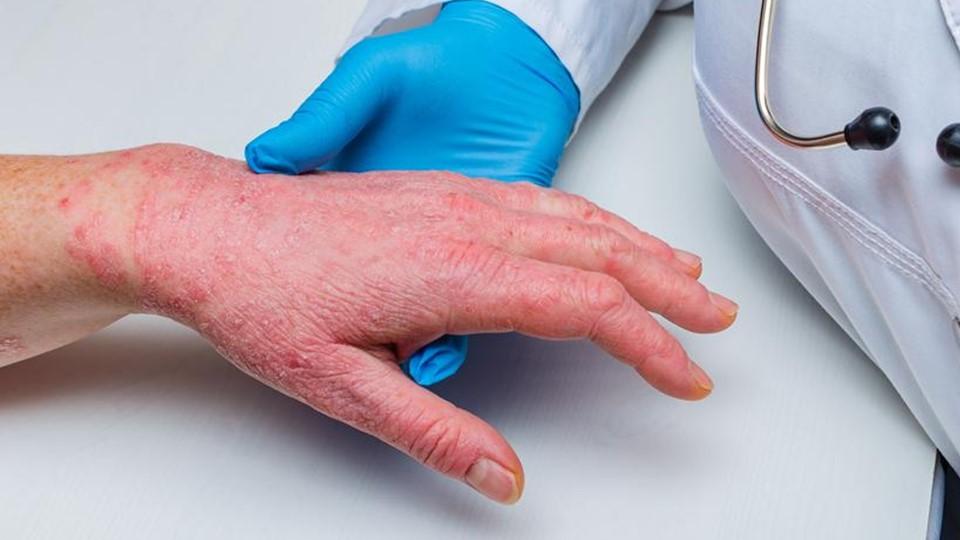
Danish dermatology specialist LEO Pharma could be just weeks away from EU approval for delgocitinib, a treatment for chronic hand eczema (CHE) considered to be its top pipeline prospect.
The EMA's human medicines committee, the CHMP, delivered a positive opinion on the drug at its meeting ahead of the weekend as a treatment for moderate to severe CHE in adult patients when topical corticosteroids are inadequate.
If approved by the European Commission – which usually follows a CHMP recommendation – the pan-JAK inhibitor will be marketed as Anzupgo in the EU and become the first topical treatment specifically indicated for CHE in this patient group.
LEO has previously said it expects Anzupgo to be a major revenue growth driver from 2025, as well as a key part of its effort to pay down debt and return to profitability, after making a loss for the last few years.
CHE is a form of dermatitis, causing symptoms like itch, cracking skin, and pain, and has been linked to a loss of integrity in the skin's barrier function, inflammation, and changes to the skin's microbiome.
Hand eczema is the most common skin disorder of the hands, with a one-year prevalence rate of approximately 9%, and is considered chronic if it lasts for more than three months or relapses twice or more often per year.
One recent survey conducted in the UK found that seven out of 10 patients felt that CHE had affected their existing relationships or ability to build new ones, while a similar proportion said it was affecting their current work or education.
For these patients, standard therapies like emollients and topical corticosteroids are often ineffective, while too much use of steroids can thin the skin, eventually making symptoms worse. There is an approved oral treatment for more severe cases of CHE – alitretinoin – but in a head-to-head clinical trial Anzupgo was more effective and better tolerated.
Alitretinoin is known to cause side effects like nausea and vomiting and is restricted to carefully controlled use in patients unresponsive to topical corticosteroids because it carries a risk of causing birth defects if taken during pregnancy.
"It is well known that CHE is challenging to live with and manage, potentially impacting personal relationships and careers," said Christophe Bourdon, LEO's chief executive.
"Despite the considerable burden of the condition, treatment options approved for CHE have been limited," he added. "As an organisation, we are dedicated to helping advance the standard of care for the benefit of people with skin conditions, and today we are one step closer to addressing the unmet need that may be faced by those living with CHE."











