Kite files CAR-T therapy in EU, ahead of Novartis rival
Kite Pharma has filed its CAR-T cancer therapy in Europe in certain types of non-Hodgkin lymphoma, hoping to get to market ahead of close rival Novartis with some impressive efficacy data.
In the US, Novartis is just ahead with its rival chimeric antigen receptor T-cell (CAR-T) therapy, tisagenlecleucel, filing it the day before Kite completed a rolling submission to the FDA at the end of March.
And last month a panel of FDA advisers unanimously backed approval for tisagenlecleucel in paediatric acute lymphoblastic lymphoma (ALL), making a green light likely in the coming weeks as the regulator usually follows the advice of its experts.
But Kite appears to have the edge in Europe after filing axicabtagene ciloleucel with the European Medicines Agency for relapsed/refractory diffuse large B-cell lymphoma (DLBCL), transformed follicular lymphoma, and primary mediastinal B-cell lymphoma.
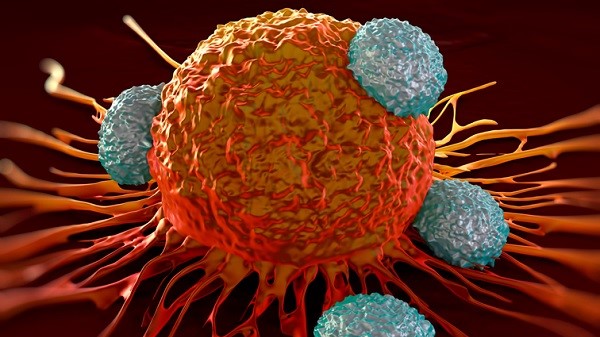
CAR-T therapies involve harvesting a patient’s own T-cells and genetically engineering them to attack targets on the surface of cancer cells.
While mid stage trials have produced strong efficacy data, side-effects are intense as CAR-T can send the immune system into overdrive with potentially fatal consequences if not managed properly.
This is also likely to drive up the already considerable treatment costs, potentially posing problems with European cost-effectiveness bodies if regulators approve the drug next year.
Kite said this is the first CAR-T therapy submitted to the EMA, in the particularly aggressive NHL types where patients often have no other treatment options.
The filing is supported by data from the ZUMA-1 trial with an objective response rate of 82% after a single infusion, with 44% remaining in ongoing response and 39% in complete response after a median follow-up of 8.7 months.
The treatment nicknamed axi-cel was developed under the EMA’s priority medicines designation (PRIME), to support the development and accelerate the review of new therapies for serious and difficult to treat diseases. This allows for a potential approval and launch next year.
DLBCL is one of the subtypes of NHL that is aggressive or fast growing. While many patients can achieve and maintain complete remission after initial treatment, patients who experience relapse or do not respond to initial treatment historically have poor outcomes. Kite estimates that approximately 7,800 patients in the five largest EU nations alone may benefit from CAR-T therapy.
Rival Juno, which is backed by Celgene, last year suffered a setback after a series of patient deaths in a trial of its lead CAR-T drug forced it to abandon development and switch to an earlier stage alternative.











