Journey slips on FDA approval of rosacea therapy
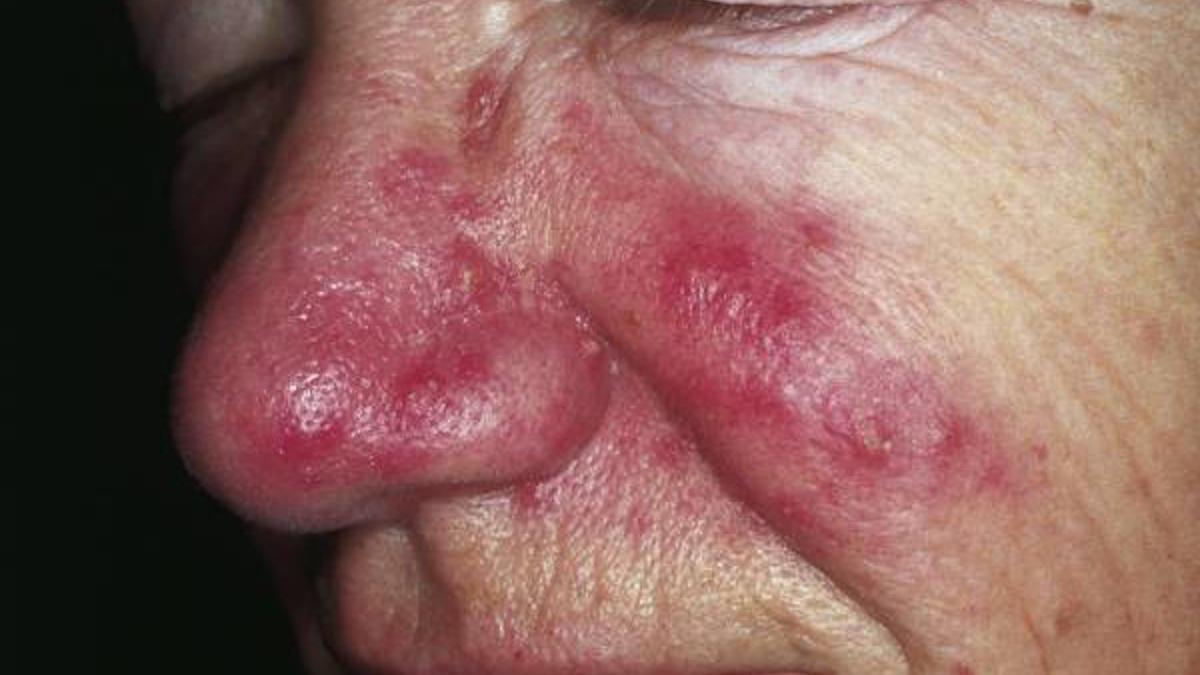
Rosacea case showing redness and inflammatory papules and pustules
Journey Medical has claimed FDA approval for its Emrosi therapy for rosacea, which it thinks could become a "standard of care" drug for the common skin disorder.
The new capsule formulation of the antibiotic minocycline hydrochloride has been cleared by the US regulator to treat the inflammatory lesions caused by rosacea – which causes flushing or long-term redness and pustules on the face – in adults.
Rosacea is estimated to affect around 16 million people in the US, said Journey's chief executive, Claude Maraoui, on an investor call held after the approval was announced, adding that Emrosi is the first new oral treatment for the condition since Galderma's Oracea (doxycycline) was launched in 2006.
Maraoui said Emrosi offers a "unique treatment option" for patients and will be launched in the first or second quarter of 2025, once manufacturing capacity has been scaled up.
Investors seemed less confident in the new drug's prospects, however, as shares in Journey fell nearly 14% after the announcement, apparently over concern about the label approved by the FDA, and slid another 6% after hours.
Specifically, there are concerns about the lack of a claim on the reduction of redness (erythema) with Emrosi, which Journey had been hoping for and would differentiate it from Oracea, which is also only approved for the treatment of inflammatory lesions in rosacea, but has become the standard treatment in the US.
Another consideration is that the first generic versions of Oracea have now launched into the US market, which could introduce downward pricing pressure.
Maraoui shrugged off those concerns, telling analysts that Emrosi "has potential to transform our dermatology business, given its anticipated strong competitive position and our existing commercial organisation," which already sells several brands including acne therapies Accutane (isotretinoin), Amzeeq (minocycline), and Targadox (doxycycline), and topical rosacea therapy Zilxi (minocycline).
The company - which recorded sales of just under $15 million in the second quarter of this year - has previously suggested that Emrosi could become a $300 million-a-year product if FDA-approved. It has not yet revealed its pricing plans for the drug but, according to Maraoui, would be in the same ballpark as the $950 per month cost of Oracea.
Journey acquired joint development and commercial rights to Emrosi (formerly DFD-29) in the US and Europe from India's Dr Reddy's Laboratories in 2021, on the back of phase 2 data. It ran a pair of phase 3 trials that showed a superior effect on Investigator's Global Assessment, as well as the reduction in total inflammatory lesion count compared to Oracea and placebo.
Image by Michael Sand, Daniel Sand, Christina Thrandorf, Volker Paech, Peter Altmeyer, Falk G Bechara via Wikimedia











