Indivior bags $111m contract from US for overdose therapy
Indivior has secured a $111 million supply contract from the US federal government for its intranasal therapy for treating opioid overdose, in a further boost to the UK company’s fortunes.
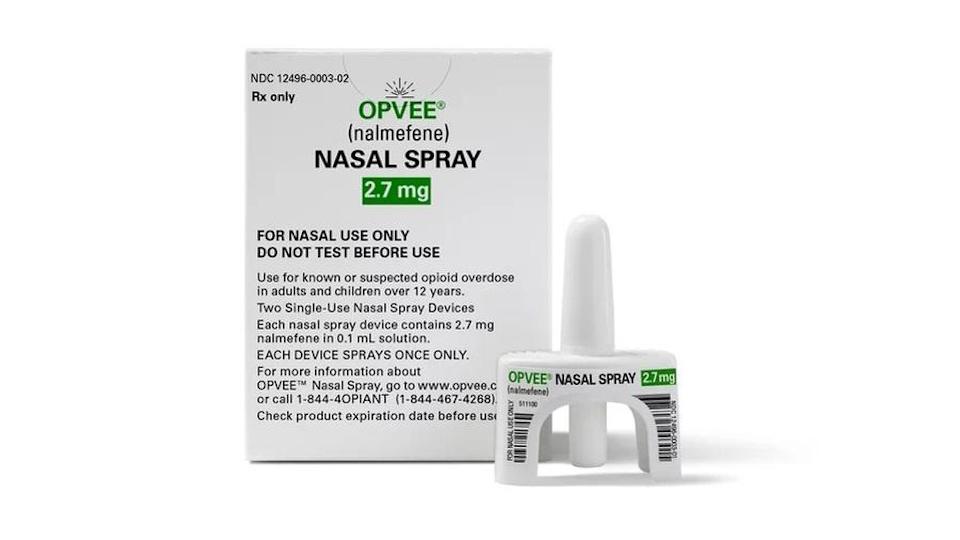
The award from the Biomedical Advanced Research and Development Authority (BARDA) will be used in part to create a stockpile of 200,000 doses of Opvee, the first nalmefene-based nasal spray to be approved for use in the US, which Indivior acquired as part of its $145 million acquisition of Opiant Pharma in February.
The FDA approved Opvee as an emergency treatment for individuals aged 12 or over who are suspected of having taken an overdose of natural or synthetic opioids. Some of the BARDA funding will be used to assist in the clinical development and regulatory approval of the drug in children under 12. The funding will be payable over nine years.
Opvee, which launched in the US in October, has been selected for the programme because it has a key advantage over naloxone nasal spray, the most widely-used overdose reversal agent in the US.
Naloxone has a half-life of around two hours after nasal administration, but the new synthetic opioid fentanyl – which is responsible for tens of thousands of overdose-related deaths every year in the US – has a half-life that is around four hours.
That means it is sometimes necessary to give a second dose of naloxone to prevent patients from slipping back into the respiratory depression and inadequate oxygenation that occurs during an opioid overdose. Nalmefene, on the other hand, has a half-life of 11 hours, extending the period of protection and doing away with the need to re-dose.
Fentanyl is 50 to 100 times more potent than morphine and was responsible for around 71,000 of the 108,000 drug overdose deaths in the US in 2021.
An Indivior spokesperson said the “proactive measure” by BARDA “signifies a necessary defence strategy in addressing the devastating consequences of the widespread fentanyl presence and aims to enhance preparedness against potential bioterrorism incidents involving this lethal opioid.”
The contract is another sign that it is emerging from a challenging few years when it has been facing allegations of improper marketing of its opioid use disorder (OUD) therapy Suboxone Film (buprenorphine/naloxone) in the US.
In October, Indivior reached a $385 million agreement to settle the last outstanding claims in the litigation, with direct purchasers of Suboxone Film in the US. That settlement came after the company paid $30 million to resolve litigation with insurers in the US in August, as well as a $103 million payment in June to resolve claims brought by more than 40 states.
It also paid out $300 million to resolve civil claims from US states in 2021, and a $600 million plea deal to settle criminal fraud charges in 2020.
Indivior reported sales of $800 million in the first nine months of 2023, a 21% increase on the same period of 2022.













