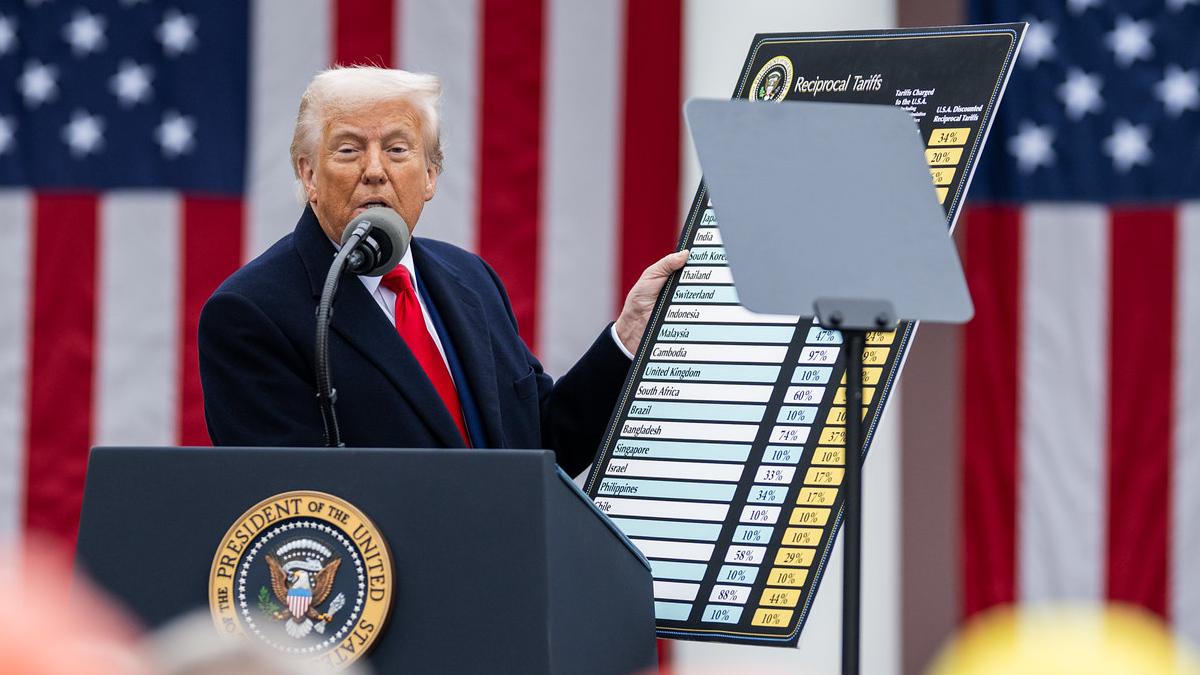
GSK takes RSV jab rivalry with Pfizer into the courts
GSK has filed a lawsuit against Pfizer in a US court alleging infringement of patents that it holds on vaccines against respiratory syncytial virus (RSV).
The suit filed in a district court in Delaware claims that Pfizer's Abrysvo vaccine infringes four patents held by GSK that cover its Arexvy shot, which became the first RSV vaccine to be approved anywhere in the world after it got a green light from the FDA in May.
Pfizer's product was approved shortly afterwards, with both vaccines indicated to prevent lower respiratory tract disease caused by RSV in individuals 60 years of age and older, in time for a rollout during the next RSV season that gets underway in the autumn.
A GSK spokesperson sent pharmaphorum the following statement confirming the lawsuit:
"In the US, GSK has initiated legal proceedings against Pfizer to enforce several patents relating to RSV vaccines. Intellectual property protections are the foundation of research-based companies' ability to drive innovation."
She went on: "This action does not impact GSK's ability to launch its RSV vaccine, Arexvy, and we remain fully focused on making our RSV vaccine for older adults available in the US following recent approval by the FDA. As proceedings are ongoing, we can't comment further at this time."
A copy of the complaint notes that Pfizer started its R&D into an RSV vaccine several years after GSK, and Abrysvo is "infringing one or more claims of the GSK patents" – cited as numbers 8,563,002, 11,261,239, 11,629,181, and 11,655,284.
"Upon information and belief, Pfizer knowingly uses GSK's claimed inventions in Abrysvo without permission," says the lawsuit. It also notes that Pfizer has previously filed oppositions to the '002 and '239 patents in Europe, claiming they are invalid.
According to the court documents, GSK is seeking an injunction on the sale of Abrysvo in the US, as well as damages, but says the company isn't seeking to prevent the use of Abrysvo to prevent RSV in infants by active immunisation of pregnant women – an indication that GSK abandoned for Arexvy after it saw signs of an elevated risk of premature birth in clinical trials.
At stake is an RSV vaccination market that has been estimated to be worth somewhere between $5 billion and $10 billion worldwide. While GSK and Pfizer are first to market, other drugmakers, including Moderna and Bavarian Nordic, are also eyeing the market with candidates in late-stage development.