FDA nod broadens use of Brainomix's stroke AI
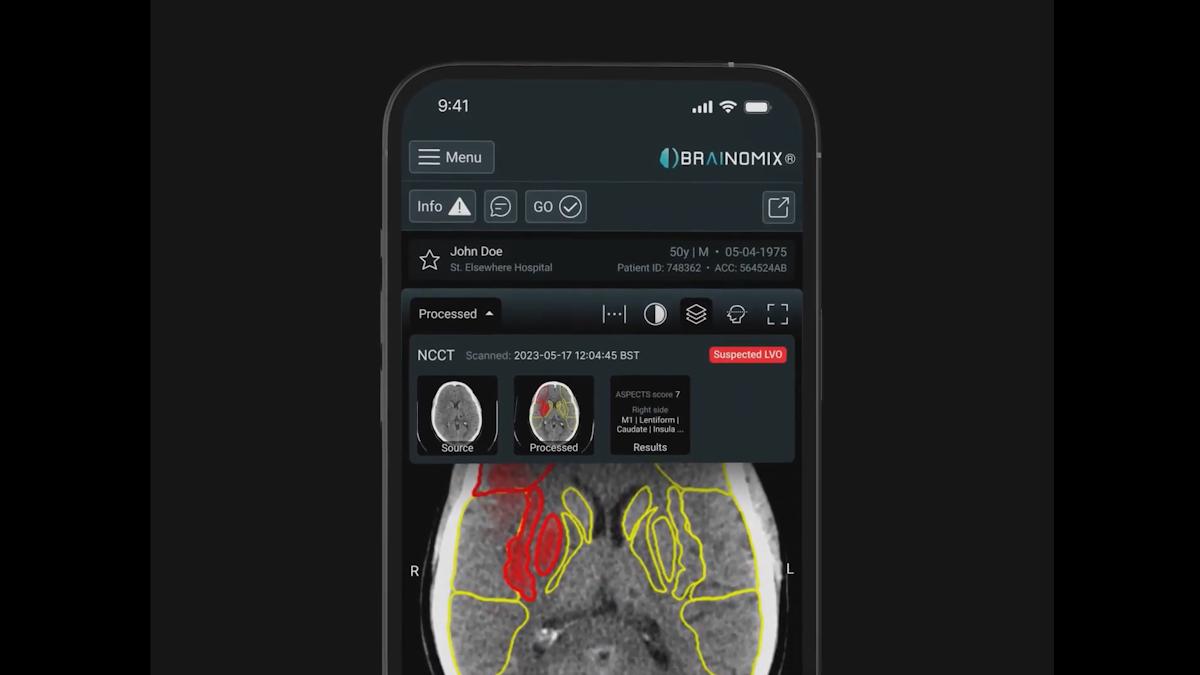
UK medtech company Brainomix has been granted FDA approval for a new feature for its artificial intelligence-powered stroke imaging software that could unlock wider use in the US.
The new approval will allow doctors to use the AI to assess ischaemic core volume – the amount of brain tissue affected by a stroke – from computed tomography (CT) scans carried out without the use of contrast agents.
That means that the Brainomix 360 Stroke software can now be used in non-contrast CT (NCCT) images, which are estimated to account for about 60% of the total number of CT scans carried out in the US every year.
Previously known as e-Stroke, the platform has been approved by the FDA for use in contrast-enhanced CTs since 2023 and supports physicians by providing real-time interpretation of brain scans to help guide treatment and transfer decisions for stroke patients.
Now, it can address a longstanding unmet need in stroke triage, enabling physicians across stroke networks to improve their decision-making for treatment and transfer of patients using routinely available NCCT brain scans, according to University of Oxford spinout Brainomix.
The new feature for assessing ischaemic core volume has been put through its paces by leading US stroke centres, with results demonstrating equivalency to CT perfusion and MRI-derived core volume assessments.
"The ability of Brainomix 360 to estimate ischaemic core volumes in a reliable and reproducible manner with a similar performance to CT perfusion represents an attractive alternative in centres without ready access to either advanced imaging modalities or stroke neurologist and/or neuroradiologist for imaging interpretation," said Dr Mehdi Bouslama, a neuroendovascular surgery and vascular neurology specialist at Broward Health in Florida, US.
The new approval "has the potential to make endovascular therapy more widely available," he added.
A study published last year in the journal Frontiers in Neurology showed that the use of Brainomix 360 Stroke enabled a primary stroke centre in the UK to triple the number of stroke patients achieving functional independence at 90 days, from 16% to 48%, and also achieved a 61-minute reduction in their door-in-door-out (DIDO) time.
Long DIDO times are an important cause of treatment delay in stroke patients and can have a big impact in how well they recover.
Last year, Brainomix's software was one of two AI tools recommended for use in the NHS to help detect stroke from CT brain scans, alongside the RapidAI platform.












