FDA approves new AI-powered colonoscopy aid
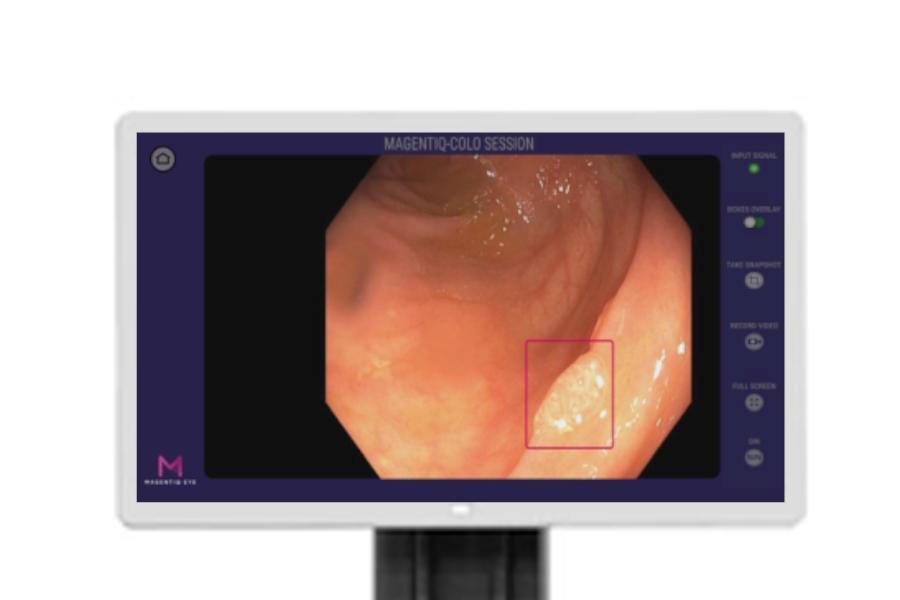
An artificial intelligence software developed by Israeli medtech company Magentiq Eye that promises to improve the accuracy of colonoscopy diagnoses has been approved in the US.
The Magentiq-Colo software detects lesions in the gastrointestinal tract in real-time and has been shown in clinical testing to improve the adenoma detection rate (ADR), a measure of how effectively doctors can spot polyps and other lesions during colonoscopies.
The FDA has cleared the AI under its 510(k) pathway for medical devices, and the green light follows approvals in 2021 in Israel and the EU. It will start to roll out in the US "in the coming weeks," according to Magentiq Eye.
In a clinical trial reported at the United European Gastroenterology Week (UEGW) congress last year, Magentiq-Colo increased the ADR by 26% compared to conventional unassisted colonoscopy, which remains the most important method of diagnosis for colorectal cancers – even though studies suggest up to 26% of lesions are missed even with skilled practitioners.
In absolute terms, the ADR went up by 7%, and according to the company, that improvement would translate into a 21% decrease in colorectal cancer occurrence and a 35% decrease in patient mortality.
The researchers concluded that computer-aided polyp detection reduced the adenoma miss rate (AMR) two-fold and was significantly better at not only detecting smaller lesions that might be missed by a doctor, but also larger, more clinically-relevant lesions.
There are 15 to 20 million colonoscopy procedures performed annually in the US, but high missed rates and undetected adenomas mean that even patients who are being regularly screened are still at risk of developing colorectal cancer, according to the Israeli company.
A missed polyp can lead to interval cancer, which accounts for approximately 8% to 10% of all colorectal cancer cases in the US, equivalent to more than 13,500 cancer cases that could be prevented every year with better detection.
"FDA clearance is a major milestone, and we are very proud to join only a handful of companies in the field of AI-aided colonoscopy to be granted clearance," said Dror Zur, founder and chief executive of Magentiq Eye.
The next step for the company will be to launch Magentiq-Colo through a local subsidiary and a network of agents, which is in the process of being set up.
"I believe this is only the tip of the iceberg for AI in gastroenterology, so stay tuned for new products and features from us coming soon," added Zur.
Other companies operating in this category include Cosmo Artificial Intelligence, whose GI Genius became the first FDA-approved AI-powered colonoscopy software in 2021, as well as Iterative Scopes' Skout and Wilson AI's EndoScreener which have also been cleared in the US.