CSL, BrightInsight launch Hemgenix companion app
CSL Behring's recently approved gene therapy for haemophilia B Hemgenix has joined the swelling ranks of new medicines that are bundled with a companion app to support the patients treated with them.
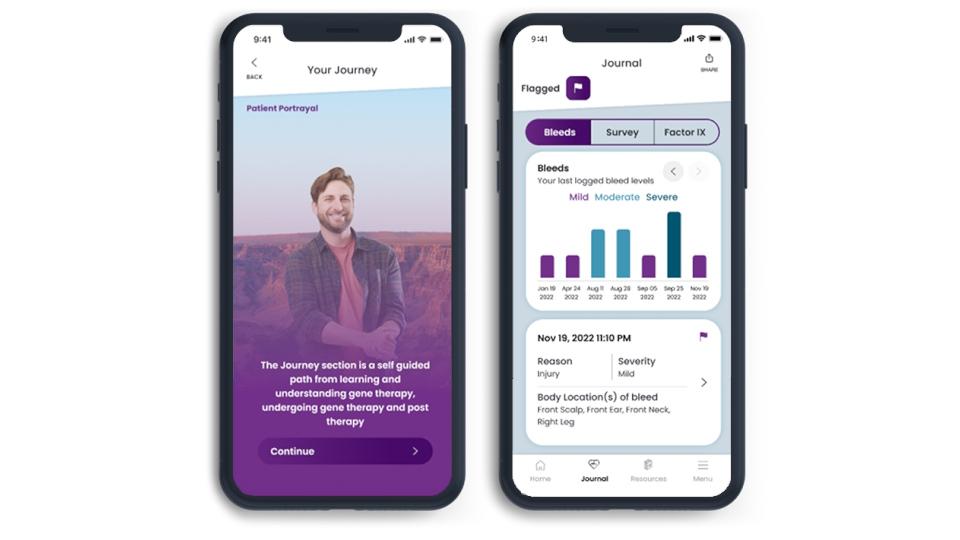
The B SUPPORT Patient app developed with companion app specialist BrightInsight is used to assess a patient's current treatment and the impact on their lives by tracking bleeds, Factor IX activity, and how they are feeling.
It also guides them through the gene therapy procedure "from eligibility to dosing to monitoring" and can be used to support patients as they make the transition from needing regular injections of Factor IX replacement therapies after the one-shot Hemgenix (etranacogene dezaparvovec) therapy.
Hemgenix was approved in the EU in February and in the US in June as the first gene therapy for adults with severe and moderately severe haemophilia B without a history of Factor IX inhibitors – antibodies that can develop in patients treated with replacement therapies.
In the HOPE-B trial, 96% of haemophilia B patients treated with a single infusion of the gene therapy had a sustained increase in Factor IX levels, which meant they could discontinue Factor IX replacement. This was accompanied by a 64% reduction in bleed rates.
"Transitioning to a one-time gene therapy is not a straight path – patients may be hesitant at trying a novel therapy," said CSL and BrightInsight in a statement on the app's launch.
The app can advise patients whether they are likely to be a candidate for Hemgenix, help them prepare for consultations with their doctors, and access a CSL support team.
"We worked closely with BrightInsight to develop an app that met the diverse needs of haemophilia B patients," said David Chu, director of marketing for Hemgenix.
"With CSL's deep market knowledge and long history serving this community and BrightInsight's astute understanding of patient needs and behaviours, we carefully studied the patient journey to identify touch points we could impact with digital. Together, we were able to build tools that guided patients through this unique journey in a very holistic way."
Future releases of the app will include additional community-focused features and support for more languages, including content and features for users earlier in the consideration phase who may not yet be aware of gene therapy as a treatment option.
This is the second companion app developed by BrightInsight to support a CSL product, after the two companies forged a digital health alliance in 2020. The first was introduced to assist patients taking Hizentra (human immune globulin) product for primary immune deficiency.













