Celyad’s CAR-T shows safe profile in early trials

Belgium-based biotech firm Celyad says trials of its CAR-T cell therapy in blood cancer patients are progressing well, with no signs of patient safety problems.
CAR-T cells, or chimeric antigen receptor T cells, are one of the most exciting new drug classes, with numerous companies developing candidates for blood cancers such as lymphoma, leukaemia and multiple myeloma.
Other companies, namely Juno, Bluebird, Kite and Novartis, have a head start over Celyad, with Novartis' CTL019 currently judged to be in the lead, but there is still much to play for.
One reason for this is safety. CAR-Ts supercharge the body's own immune T cells to target and kill tumours, and could transform outcomes for patients.
However by unleashing this power, the drugs risk serious side effects; in the worst cases, a potentially fatal inflammatory response, called a 'cytokine storm', can be triggered.
In particular, six deaths linked to this response caused Memorial Sloan Kettering and Juno to halt their trials in 2014. These have now been resumed, but the incidence of life-threatening fevers and inflammation still occur in up to one third of patients given CAR-T treatment.
Celyad's candidate NKG2D is different to many of its rivals, which are developing CD19 CAR-Ts that only target one tumour antigen. NKG2D uses natural killer (NK) cells instead, which have the potential to target a broad range of cancers.
NK cells should also be safer. They have a limited life span inside the body, unlike CD19 CAR-Ts, which can remain in circulation for longer, potentially causing inflammation.
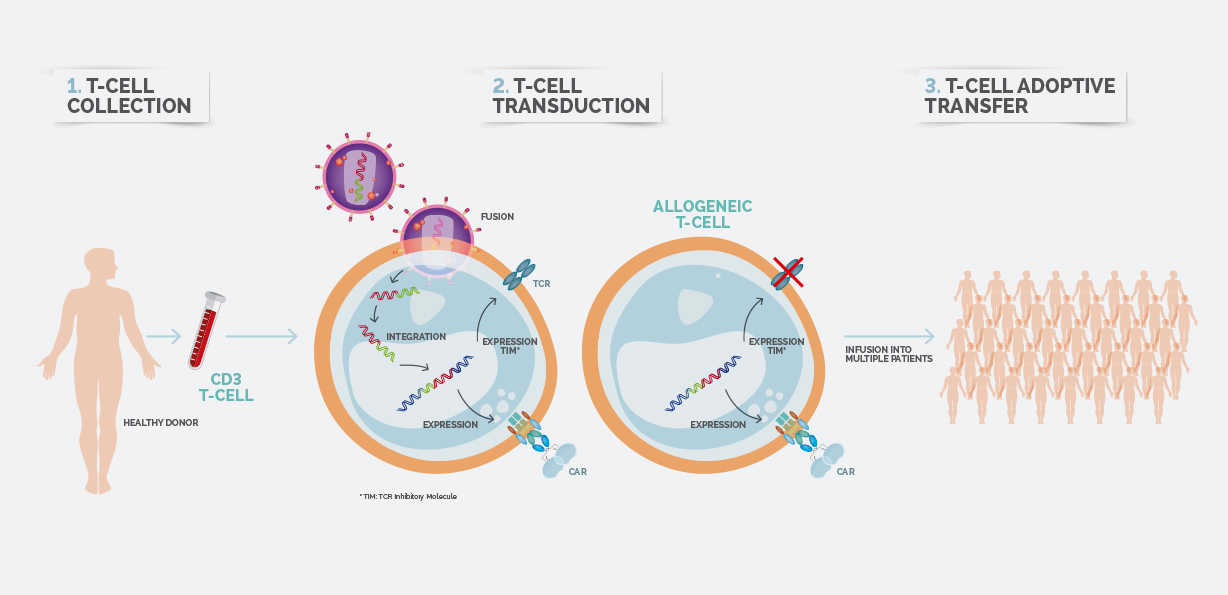
Celyad's allogeneic platform produces 'off the shelf' CAR-T cells. Image: Celyad
NKG2D has another advantage – it uses allogeneic technology, allowing 'off-the-shelf' CAR-T therapy to be produced more quickly than the other drugs. This approach is something Celyad has in common with France's Cellectis, whose candidate UCART19 has caused considerable excitement in recent months.
Phase I/II trial proceeds
Celyad has announced today that a 30-day safety follow-up on the first patient enrolled in the second cohort of its phase I trial has produced no worrying signals.
The small phase I/II trial is in patients with acute myeloid leukaemia (AML) or multiple myeloma (MM).
The trial is designed to assess the safety and feasibility of NKG2D CAR-T cells, with secondary endpoints including clinical activity in these haematological indications.
Dr Christian Homsy, chief executive of Celyad, said the results built on earlier encouraging safety news, adding that recruitment for the trial would continue.
Data readouts from the first 12 patients treated in the phase 1 portion are expected in mid-2016. Once the recommended dose is determined, the IIa phase of the trial will enrol 12 additional patients.
Founded in 2007 as Cardio3 BioSciences, the firm had an active 2015, floating on the stock market and changing its name to reflect a broader scope across innovative cell therapies. Its lead candidate is C-Cure, a novel cell therapy for heart failure which is expected to produce preliminary phase 3 results in mid-2016. The company only entered the CAR-T field in January when it acquired privately-held US biotech OnCyte in January, subsequently changing its name to reflect its move beyond the cardiovascular field.
It also launched its IPO on the NASDAQ exchange in June, which raised over $100 million to fund the business. In September GlaxoSmithKline's head of early-stage cancer immunotherapeutics, Dr Frédéric Lehmann, joined the firm as head of Immuno-oncology.










