Astellas taps Eko digital stethoscope for heart failure DTx

Astellas has licensed rights to a smart stethoscope device developed by Eko Health, that it will use as part of a digital therapeutic (DTx) approach to heart failure.
The plan is to combine the CORE 500 digital stethoscope and artificial intelligence algorithms used to detect and diagnose cardiovascular disease with a digital health platform developed by Welldoc that is used to connect patients, care teams, and healthcare systems.
The combined DTx and device product – codenamed Z1608 – will be used for remote monitoring of physiological biomarkers associated with heart failure in patients, and provide coaching on diet, activity, and medication adherence, with content also provided by the American Heart Association (AHA).
Ultimately, the goal is to show that Z1608 can reduce the frequency of acute decompensation events in patients living with heart failure and to secure FDA approval for that application.
Last year, Eko got FDA approval for the CORE 500 device – which combines a stethoscope with a three-lead electrocardiogram (ECG) – which can pick up cardiovascular abnormalities such as heart murmurs, atrial fibrillation (AF), and low left ventricular ejection fraction (LVEF) in seconds during routine physical examinations.
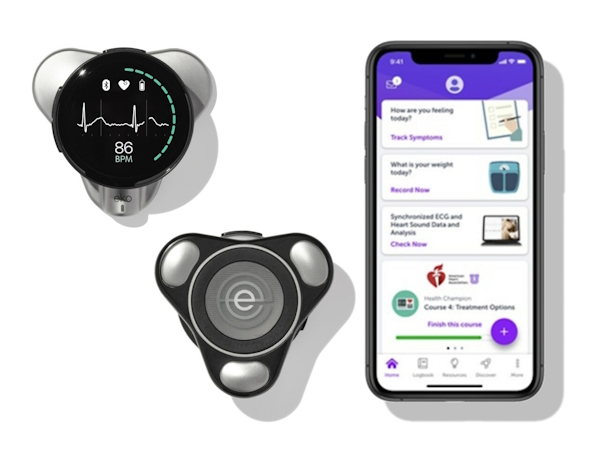
The accompanying AI algorithm is also cleared by the FDA to detect murmurs and AF, and last year an investigator-led study showed a simpler Eko device – with a single-lead ECG – was able to detect LVEF with an accuracy of 80% to 90%. LVEF is seen in around half of all heart failure patients.
Welldoc has been working with Astellas on digital health projects since the two companies entered into a strategic alliance in 2019 that initially focused on diabetes, but also covers other therapeutic categories, including heart failure, hypertension, and pre-diabetes.
So far, only the diabetes functions of the app are FDA-approved, but Astellas said Welldoc offers “established cardiometabolic DTx capabilities.”
There is a high unmet medical need for a non-invasive, connected, portable, and user-friendly digital solution for supporting patients living with heart failure, said the partners in a joint statement.
“Caring for heart failure patients at home requires the ability to precisely measure patient data,” said Connor Landgraf, co-founder and chief executive of Eko.
With Z1608, “we’re closer to a future where heart failure is proactively managed, rather than reactively treated […] and bridges the home-clinic care gap” he added.













