AmerisourceBergen adds Parkinson’s app to DTx platform
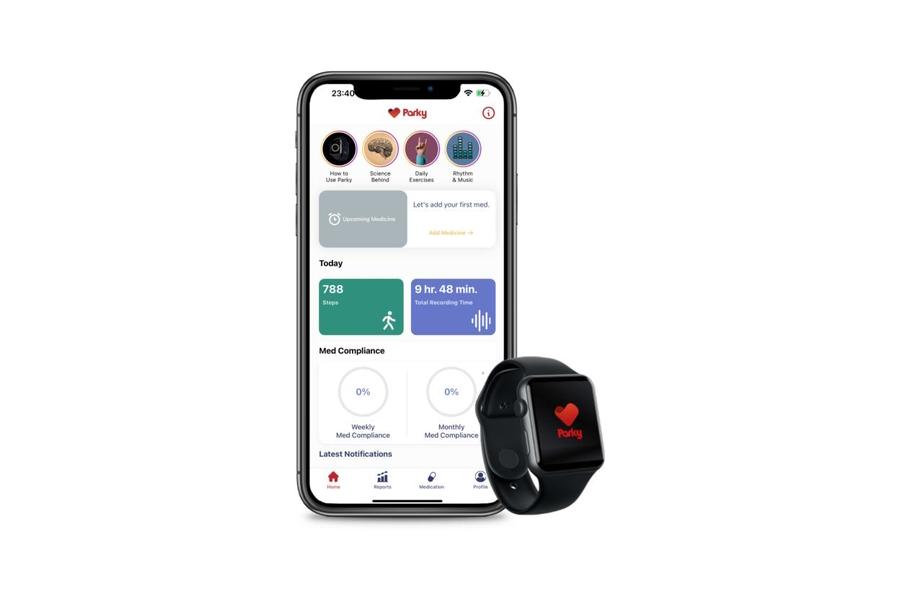
h2o Therapeutics will soon be able to call on the marketing reach of pharma distributor AmerisourceBergen for its app for patients with Parkinson’s disease.
The Turkish digital health company received FDA approval for the Parky app last November as a prescription digital therapeutic (DTx), used to monitor symptoms such as tremors and dyskinesia in Parkinson’s patients in real-time using the Apple Watch.
That came just a few months after AmerisourceBergen launched DTx Connect, a system designed to help physicians order prescription and non-prescription DTx products and handle dispensing and fulfilment. Now h2o has joined other DTx players like Mahana Therapeutics and Videra Health by making its app available on the platform.
Parky was approved on the back of a study in 343 subjects, which demonstrated the feasibility of using the app to track patients over six months using the Apple Watch’s inertial sensors and Apple’s Movement Disorder API.
The study – published in the journal Science Translational Medicine in 2021 – found that the app matched clinician-reported evaluations seen during in-clinic visits, suggesting it could be used to spot changes in symptoms that could help guide treatment decisions, as well as support clinical trials of new Parkinson’s therapies.
AmerisourceBergen’s move into the digital health area is part of a revamp of the business, which will be renamed Cencora later this year to reflect a change in business strategy, with international expansion as well as a move beyond its traditional focus on drug distribution.
The company has been expanding its scope in recent years, notably with the $6.3 billion acquisition of Walgreens Boots Alliance’s Alliance Healthcare businesses in 2021, which boosted its distribution business in Europe, as well as smaller deals like its $1 billion takeover of healthcare and life sciences consulting and outsourcing provider Pharmalex earlier this year.
Digital health is a key part of its focus, and the company said at the time it launched DTX Connect that the US market for digital therapeutics was worth $4.2 billion in 2021 and predicted to grow at a compound annual growth rate of nearly 25% between through to 2030.
Since then, however, there have been some setbacks for the sector, headlined by the problems besetting Pear Therapeutics, a DTx pioneer that has struggled to make a viable business despite having three FDA-approved products.
For h2o, the hope is that having access to the DTx Connect platform will make it easier to build a market for Parky in the US. The app is being made available initially on a pilot basis.
 “Following the recent FDA clearance, we continue to focus on strategies and solutions that will enable us to expand patient access to Parky,” said Yağmur Selin Gülmüş, h2o’s founder.
“Following the recent FDA clearance, we continue to focus on strategies and solutions that will enable us to expand patient access to Parky,” said Yağmur Selin Gülmüş, h2o’s founder.
“Through this arrangement with AmerisourceBergen, our goal is to reduce barriers along the patient journey, improve access and expand our commercial reach,” she added.
The company has two more digital therapeutics products in the pipeline based on wearable devices, and FDA submissions are planned to be completed in 2023.
Those are Foggy, an app intended to support people with neurodegenerative diseases, and Covie, a COVID-19 early detection system.












