AliveCor pledges to outdo Apple Watch with 6-lead ECG

Apple made headlines earlier this month with its announcement of a new watch with an FDA-approved electrocardiogram (ECG) reader to indicate the wearer’s cardiac health.
But not to be outdone, AliveCor, which had previously produced a competing ECG-on-a-phone smartphone app and reader device has pledged to introduce a more accurate six-lead competitor in the coming months.
Apple launched its new product shortly after receiving FDA clearance, and can detect if a person’s heart rate is too low or is going into atrial fibrillation.
But AliveCor is hitting back, after already getting its product FDA-approved to detect high potassium levels in the blood.
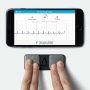
AliveCor's smartphone ECG
Now the TechCrunch website reports that AliveCor wants to compete with Apple by providing a “never-before-seen” six-lead ECG reader smartphone app.Vic Gundotra
The number of ECG leads can vary, with more leads producing more accurate results. In clinics patients can have up to 12 leads or stickers placed across their chest to pick up cardiac data.
Apple’s system has just one lead, but the six-lead system could be more accurate and pick up more information.

Apple Watch
AliveCor CEO told TechCrunch the six-lead device could pick up about 100 different diseases.
This would importantly include ST elevation, a key factor associated with the onset of a heart attack, which could get a person on their way to hospital before they begin to display physical symptoms.
Gundotra was reportedly unfazed by the suggestion that Apple could develop its own competing six-lead ECG system.
He told TechCrunch: “They could but we have some pretty good patents in the space.”
AliveCor will be talking to the FDA to decide which regulatory pathway to use to get the as yet unnamed device to over-the-counter consumers by 2019, according to the report.











