AbbVie gets continuous Parkinson's drug over the line in US
AbbVie has finally claimed FDA approval for its advanced Parkinson's disease therapy Vyalev, 18 months after the US regulator turned it down with a request for more data.
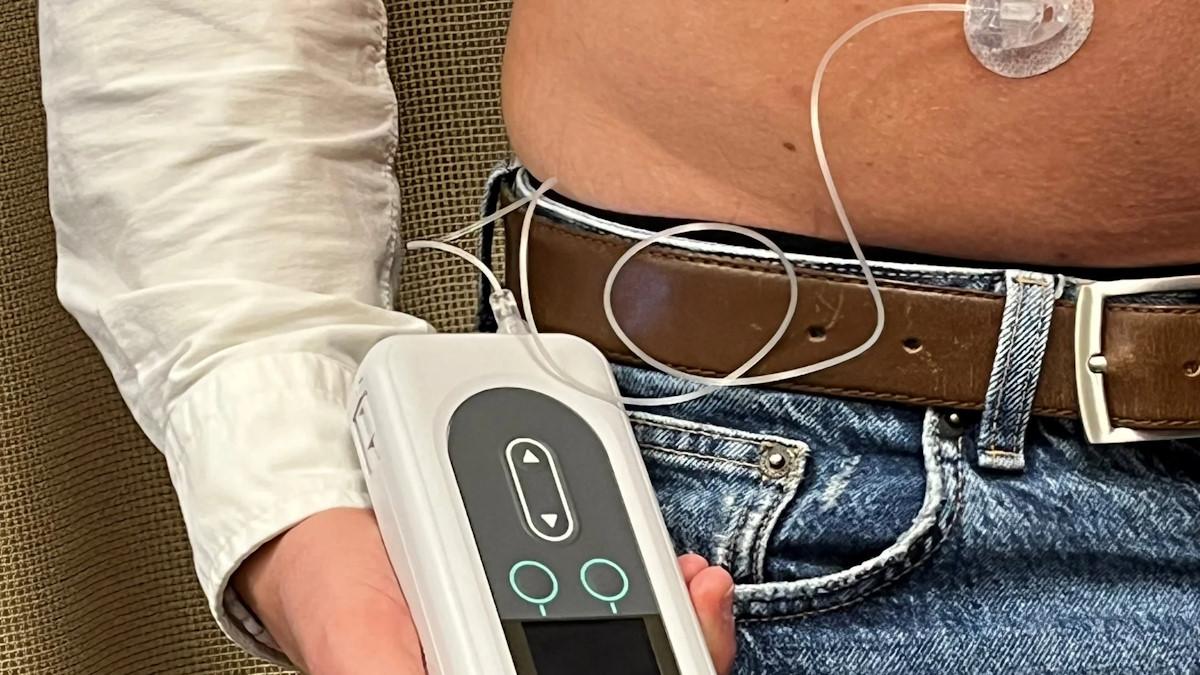
Now, Vyalev (foscarbidopa and foslevodopa) has become the first subcutaneous 24-hour continuous infusion of levodopa-based therapy for the treatment of motor fluctuations in advanced Parkinson's, delivered via a pump.
Questions about the pump used in the product led to the FDA's decision to reject the marketing application in March 2023, but Vyalev will soon be an option for patients in the US who struggle to control symptoms throughout a 24-hour period using conventional oral Parkinson's therapies.
AbbVie said in a statement that the timing of patient access to Vyalev is "dependent on their individual insurance plan," adding that it expects Medicare coverage to start "in the second half of 2025."
In a clinical trial, patients treated with Vyalev (formerly known as ABBV-951) had a statistically significant increase in patient 'on' time without dyskinesia – in other words, the period where muscle symptoms associated with Parkinson's are controlled without involuntary, uncontrolled movements – compared to treatment with immediate-release carbidopa/levodopa.
The drug also achieved a significant reduction in 'off' hours when symptoms re-emerge versus the oral drugs, and was generally well-tolerated with the most common side effects reported being infusion site reactions, hallucinations, and dyskinesia.
Vyalev is already available in the EU, where it has been approved under the Produodopa brand name since 2022. AbbVie held off on launch until it also got the green light from the European Commission for its portable Vyafuser pump device – developed in partnership with Phillips-Medisize – which came through last November. The EU launch kicked off in January.
The Vyafuser pump maintains a continuous, steady flow of the drug throughout the day and can be refilled at home by patients or their carers.
"People living with advanced Parkinson's disease experience daily challenges as a result of uncertainty in managing motor fluctuations, especially as their disease progresses," remarked Roopal Thakkar, AbbVie's chief scientific officer.
"We are proud to bring this innovation to patients who may benefit from motor symptom control through continuous 24-hour administration of Vyalev," he added.
The product has also been cleared for marketing in GB and endorsed for NHS use in England and Wales by health technology assessment (HTA) organisation NICE.
AbbVie also sells another long-acting therapy for advanced Parkinson's called Duodopa (levodopa–carbidopa intestinal gel) which has been bringing in around $500 million a year in sales.
Duodopa is however administered as a continuous infusion using a portable pump, via a percutaneous jejunostomy tube, which requires a surgical procedure to set up, so is expected to be superseded by Vyalev, which some analysts have predicted could become a $1 billion-plus product.
It could face competition from other subcutaneous infusion therapies, notably Mitsubishi Tanabe Pharma's ND-0612 which is in late-stage clinical testing.












