How can we speed diagnosis of rare diseases?

Wendy White of Siren Interactive explores the challenge of achieving a diagnosis in the rare disease space and the collaborative initiatives underway to tackle this challenge.
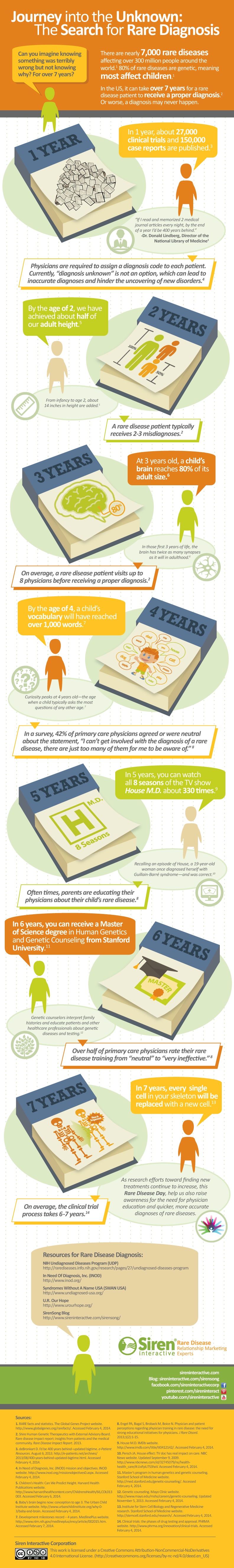
Getting the right diagnosis is one of the greatest challenges facing people with rare diseases and their families. According to the Shire Rare Disease Impact Report, it takes on average 7.6 years in the US and on average 5.6 years in the UK for patients with a rare disease to receive a proper diagnosis; along the way they often receive two to three misdiagnoses.
Even more striking is the human cost of this long journey to diagnosis. More than 50% of rare disease patients are children and the time lost can result in developmental delays that could have been avoided, organ damage, and even death. Patients who are misdiagnosed may receive inappropriate treatments and surgeries that can harm them further. Perhaps most painful of all is the heartbreak of parents whose child dies undiagnosed, after years spent going from specialist to specialist in search of a name for the disease.
Time is of the essence. New treatments for rare diseases are being developed every day, but without a proper diagnosis patients can't benefit. It's critical that we solve the complex problem of speeding diagnosis.
Collaboration is key
Meeting this challenge is going to require a multi-faceted approach. Rare communities and orphan drug companies discovered long ago something that the rest of the pharmaceutical industry is now beginning to understand as well—the way to make progress is to collaborate. This is particularly true for diagnosis.
We know many of the pieces that should be involved in speeding diagnosis, but also their limitations. Educating physicians will help, for example, but it's unreasonable and impractical to expect physicians to become experts on 7,000 rare diseases that they may only see once, twice, or perhaps never in their day-to-day practice.
Early referral to specialists will help as well, but it involves yet more education for physicians on how to target referrals. A recent study published in the Journal of Rare Disorders found that patients saw an average of 7.3 physicians before a correct diagnosis was made.
The good news is that collaboration is beginning to happen and progress is being made. Patient organizations and the National Institutes of Health (NIH) are adding more initiatives to help the undiagnosed. Pharmaceutical companies and biotechs are lending their support to community awareness initiatives, new technologies are being leveraged, and rare patients and caregivers are becoming experts themselves.
Organizations and government programs are supporting diagnosis
Patients and caregivers are working on many different fronts to improve the diagnosis of rare disease. The National Organization for Rare Disorders (NORD) is focusing on educating healthcare professionals with a series of physician guides. They currently have a dozen of these disease-specific guides, with more in progress.
Global Genes recently enhanced their offerings to help undiagnosed patients by partnering with SWAN USA (Syndromes Without A Name) to offer free clinical genomic sequencing testing to rare disease patients who cannot afford costly tests. The first 30 patients will begin testing March 1, 2014.
In Need of Diagnosis and U.R. Our Hope are two other organizations that provide education, advocacy, and support for people seeking a diagnosis. Heather Long, a co-founder of U.R. Our Hope, is also working to interest legislators in a bill that would require the National Institutes of Health (NIH) to create an undiagnosed research and collaboration network.
There are already some new initiatives underway at the NIH. Five years ago, the NIH created an Undiagnosed Diseases Program to provide help and support to people with a longstanding medical condition that eludes diagnosis by a referring physician. They are preparing to expand that program and will be announcing a network of sites at teaching hospitals around the country.
One of the most successful and low-cost improvements in diagnosis is newborn screening. State public health labs screen some 4 million babies in the US every year for more than 50 disorders, and many bills are pending in state legislatures to add more. Approximately one in every 300 newborns has a condition that can be detected through screening. The Newborn Screening Reauthorization Act passed the Senate on January 29, 2014 and now goes to the US House of Representatives for consideration.
In Europe, Orphanet provides a robust online portal to assist physicians and patients with all aspects of rare diseases including diagnosis. Their search tool allows physicians to retrieve information about diseases by searching clinical signs using a controlled vocabulary organized by main organs and systems.
What new technologies may offer
New technologies represent one of the most exciting new frontiers that have been explored to support physicians in their efforts to speed rare disease diagnosis.
FindZebra, a rare disease search engine, was launched in March of 2013. The project grew out of a collaboration with the University Hospital in Copenhagen, Denmark. It was designed as an alternative to PubMed and Google searches which are too broad to be helpful in the specialized world of rare diseases. It's proving to be easy to use and useful even to nonprofessionals.
FindZebra helped Ed Fennell, a caregiver for his granddaughter, become interested in new technologies. "I took out the pediatric neuro's report from when Hayley was 4 months old, plugged the results into FindZebra and CDKL5 came up in the top three, along with Rett's," Fennell says. It had taken doctors nearly 3 years to arrive at the same diagnosis. Fennell was convinced that easily available technology was key. To accelerate development of an app with an easy-to-use interface that would allow doctors to enter symptoms and get suggestions on possible diagnoses, Fennell helped organize a hackathon at Rensselaer Polytechnic in Albany, NY last year.
Author and geneticist Sharon Moalem, MD, PhD has taken yet another approach. He's spent the past 7 years developing an inexpensive facial recognition tool for congenital disorders that leverages technology developed for the security industry. This tool will help physicians screen for rare genetic conditions that would otherwise be missed.
As part of a year-long focus on diagnosis, Siren Interactive will be partnering with Dr. Moalem and the Global Genes Project, a leading rare disease patient advocacy organization, to create a rare disease track at Healthcare's Grand HackFest with H@cking Medicine at MIT on March 14-16. Full details on the hackathon can be found in our pharmaphorum post for Rare Disease Day, February 28, 2014.
"Rare patients lead the way in their use of social media to connect with others in their communities."
How pharma can participate
Rare disease patients, caregivers, and healthcare professionals consider pharmaceutical companies as members of the community. They have a mutual interest in improving speed to diagnosis. They also welcome industry participation in raising awareness and providing needed education on these often overlooked diseases.
Many of our pharma clients already support speeding diagnosis by engaging the community in educating others with shareable tools, resources, and awareness campaigns. DiagnosingAIP is a good example of an online resource increase understanding among physicians about a disease that is often misdiagnosed. Acute Intermittent Porphyria (AIP) presents with a wide variety of symptoms and can only be diagnosed during an attack, so it is often mistaken for more common disorders. The website was created to address the need for education by offering a variety of professional resources developed in partnership with emergency room physicians.
Rare patients lead the way in their use of social media to connect with others in their communities. Because the journey to diagnosis is often long and frustrating for these patients, they are eager to help others who are traveling the same road. Other entities—from pharma companies to healthcare professionals—can help tremendously by bringing any of the players required to speed diagnosis together on the same pathway to make the progress we need, and to make it soon.
The only way we'll get there is together. No one understands this quite as well as a rare disease patient or caregiver
For more facts on rare disease diagnosis, view and share our infographic, Journey into the Unknown: The Search for a Rare Diagnosis.
About the author:
Wendy founded Siren Interactive in 1999. For more than 14 years across more than 30 disease states Siren has had 1 focus, finding rare disease patients and connecting them to appropriate therapies. A recipient of the 2011 Manny Heart Award, and featured on the Inc. 5000 list of fastest growing privately held companies for the past four years, Siren Interactive has been a trailblazer in recognizing the shift in patient driven decision making and innovating to meet patients and caregivers where they live. She served as a board member for the National Organization for Rare Disorders (NORD) for four years, is a current board member and the President-Elect for the Healthcare Businesswomen's Association (HBA), a board member of Global Genes, part of the Chicago Life Sciences Consortium executive advisory group and a trustee for the Boys & Girls Club of the Union League of Chicago.
Wendy approaches the business from her unique perspective of being an entrepreneur and digital marketing expert, as well as being the mother of a daughter with a rare disorder that was diagnosed as a direct result of Wendy becoming an empowered caregiver. Wendy's personal journey with her daughter, which began in 2001, was the impetus for Siren Interactive to focus solely on marketing rare disorder therapies. In 2011, Wendy led the collaboration with 12 fellow rare disease caregivers to co-author Uncommon Challenges; Shared Journeys: Stories of Love, Hope and Community by Rare Disease Caregivers. In 2012 she was recognized as one of the 100 most inspiring people in pharma.
Wendy received a bachelor's degree in quantitative business analysis from Indiana University.
For more detailed information please view Wendy's LinkedIn page
Closing thought: How can we speed up diagnosis in rare diseases?












