When health outcomes assumptions meet real patients

As the pharma industry reviews data presented at the latest ISPOR meeting in New Orleans, the Kantar Health Health Outcomes group reveals some interesting research that suggests self-reported patient data could hold the key to making the right health economic assumptions.
As an industry, we have been discussing the importance of conducting robust health economic analysis around new drug launches for a while now, particularly with regards to markets with robust heath technology appraisal processes in place, such as the UK.
However, in 2013, it feels like we have passed a point of no return, with more and more physicians, payers and even patients wanting to understand the real-world evidence for new medicines. There is a genuine desire in all geographies to understand the outcomes of interventions beyond the clinic.
The challenge today is therefore all about accurately predicting the outcomes for new medicines before they come to market and then rapidly analysing the real-world impact on patients once they are approved. In conducting such analysis a number of assumptions exist, which should try to take account of important factors such as:
• Disease burden
• Level of, and success of, diagnosis
• Patient demographics in the real-world
• Presence and level of comorbidities
• Associated medications and possible drug interactions
• Patient adherence to medicines
• Definition of beneficial outcome (e.g. management of symptoms versus quality of life)
The question is, how often this happens in practice? Also, even when it does do we really have the right health outcomes data available to make such assumptions?
We wanted to test some of these assumptions at the recent International Society for Pharmacoeconomics and Outcomes Research (ISPOR) event in New Orleans this year, where over 3,000 health economic experts were gathered from all over the world. During the event, attendees were chosen at random and presented with the following statements, to which they were asked to respond with 'Disagree', 'Neutral' or 'Agree':
1. Disease burden may differ when comparing diagnosed and undiagnosed patients with symptomatic diseases.
2. Health outcomes can be driven by comorbidity burden.
3. More convenient therapies, e.g. orals versus non-orals, may improve health outcomes.
Why not answer them yourself and write down whether you agree, disagree or are neutral on each statement before checking out how others responded?
"Do we really have the right health outcomes data available to make such assumptions?"
The collated results from the attendees were then compared with real outcomes data from the Kantar Health National Health and Wellness Survey, the largest self-reported patient database in the healthcare industry. The results from both the attendees and the associated analysis from the patient database are available in this fully accessible white paper, first published on pharmaphorum, but without giving the whole game away the results certainly proved interesting!
For example, take a look at figure 1 for some statistics on how differential the outcome is for adherent versus non-adherent patients, as derived from real data, and see if you can guess the therapy area (it might surprise you)...
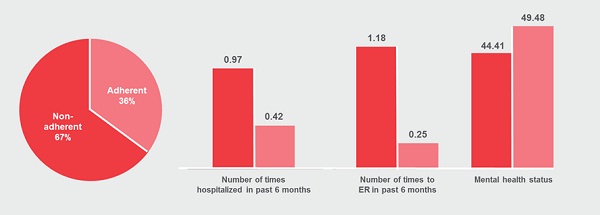
Figure 1: The level of non-adherence and impact on treatment outcomes for an anonymous therapy area (revealed in the white paper!).
What caught our attention was that, even in those questions where there was more consensus amongst respondents, the degree to which the factors mentioned impacted the outcomes on real patients (as assessed by the self-reported patient data) was surprising, even to seasoned health outcomes experts.
"Extrapolating clinical trial outcomes into the real-world is fraught with pitfalls"
Overall, this study presented a number of interesting findings which can be summarised as:
• Even where we assume real-world factors impact patient outcomes, the level of bias is often surprising.
• The outcomes we perceive as being important from a clinical perspective may not match those which patients perceive as important.
• Extrapolating clinical trial outcomes into the real-world is fraught with pitfalls.
• Patient-reported data is invaluable in helping to predict outcomes or, at least challenge our assumptions.
We believe that accurately forecasting outcomes in the real-world has become the new currency of success but, like any forecasting model, the assumptions that are fed in are as important as the model itself.
The key lesson here is that if you are not sure about these assumptions, then ask the patient!
Download the full white paper via Kantar Health here.
About the authors:
Gary Gatyas, is Senior Vice President, Global Marketing and Communications, for Kantar Health. His role includes leading the healthcare consultancy's global brand, marketing communications, media and public relations programs.
For more information, about Kantar Health and the National Health Wellness Survey, email nhws@kantarhealth.com.
Do patients understand health outcomes better than anyone?











