Humanized FcRn Mice: promising tools for evaluating the pharmacokinetics of monoclonal antibodies

Ray Lambert
The Jackson Laboratory
Ray Lambert looks at the potential of humanized FcRn mice for evaluating the pharmacokinetics of mAbs.
Due to their extraordinary specificity and pharmacokinetic (PK) behavior, human immunoglobulin gamma monoclonal antibodies (hIgG mAbs) are a most promising biological therapy for treating an expanding number of human diseases. However, before they can be FDA-approved, therapeutic hIgG mAbs must be evaluated in preclinical trials using non-human animal models. Although primates are the closest non-human surrogates for evaluating the PKs of therapeutic mAbs, they are expensive and encumbered by ethical constraints. Unfortunately, conventional rodent models do not reliably predict the PK behavior of hIgG mAbs in humans. The major reason for this non-concordance is species-specific differences in the affinity of the receptor responsible for controlling the PK of IgGs – the "Fc receptor, IgG, alpha chain transporter" (FCGRT, common name FcRn). Humanized FcRn mice – mice deficient for mouse FcRn (mFcRn) but transgenic for human FcRn (hFcRn) – circumvent this limitation. Analyses of humanized FcRn mice are increasingly documenting their predictive value in modeling the PK behavior and efficacy of therapeutic hIgG mAbs in humans.
 ,
"Unfortunately, conventional rodent models do not reliably predict the PK behavior of hIgG mAbs in humans."
 ,
FcRn
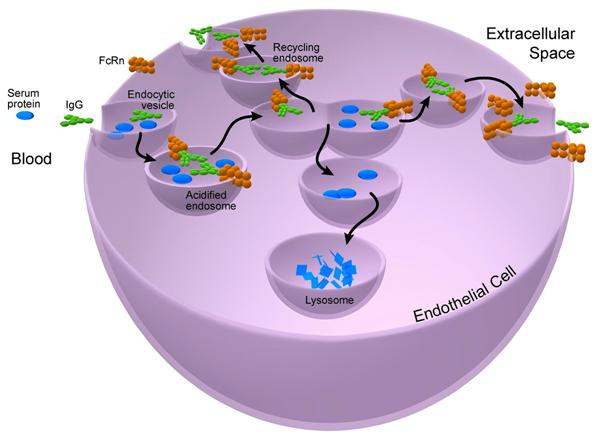
The MHC class I-like molecule FcRn is an intracellular trafficking receptor with diverse and critical immune system functions.1-3 It is unique in being able to extend the half-life (a measure of PK behavior) of IgGs in the circulation and extravascular sites. It does so by binding to the IgG-Fc protein domain. This binding is a low pH-dependent process and occurs in the acid environment of early epithelial cell endosomes. Thus bound, IgGs are protected from lysosomal degradation and can be recycled to the extracellular environment, increasing their bioavailability (Figure 1). Because of its critical role in controlling the PK of IgGs, FcRn has been exploited to design therapeutic mAbs and related biologics.
Figure 1. FcRn rescues IgGs.
IgGs and other soluble proteins in extracellular fluids are endocytosed into acidic endosomes by vascular endothelial cells. FcRn, an integral endosome membrane protein, binds to IgGs and recycles them either into the circulation or the extracellular space (both pH neutral), where they are released. The other proteins are channeled to the lysosomes and degraded.
FcRn Binding to IgG Differs Among Species
FcRn from one species may or may not bind to / protect IgGs from another. For example, mouse IgGs (mIgGs) don't bind to hFcRn, but both mIgGs and hIgGs bind to mFcRn. In fact, hIgGs have a very high affinity for mFcRn. As a result, their half-lives in conventional mice are unrealistically long. Because humanized FcRn mice express only hFcRn, they can be used to more reliably assess the therapeutic efficacy of candidate hIgG mAbs.
FcRn Mice: Properties and Capabilities
Properties of FcRn mice
• Each FcRn strain has a C57BL/6J genetic background, reducing the biological noise caused by the genetic variation of mixed backgrounds.
• Humanized FcRn mice have minimal abnormalities other than low endogenous serum IgG concentrations.4
• The FCRN transgenes affect the PK behavior of hIgG mAbs in a copy number-dependent manner: two copies (instead of one) increase the serum half-lives.5,6
• hIgG mAbs generally have shorter serum half-lives in cDNA FCRN transgenic mice than in humans, half-lives are longer In genomic FCRN transgenic mice, approaching that of humans.5,6
Suitabilities for evaluating therapeutic mAbs
• The cDNA FCRN transgenic strain B6.Cg-tm1Dcr Tg(CAG-FCGRT)276Dcr/DcrJ is most sensitive for detecting subtle differences in mAb PKs.
• The genomic FCRN transgenic strain B6.Cg-Fcgrttm1Dcr Tg(FCGRT)32Dcr/DcrJ is best suited for modeling the greatly extended serum half-lives of hIgGs observed in humans.
• Rag1 immunodeficient cDNA FCRN transgenic strain B6.Cg-Rag1 tm1Mom Fcgrttm1Dcr Tg(CAG-FCGRT)276Dcr/DcrJ and Scid immunodeficient genomic DNA FCRN transgenic strain B6.Cg-Fcgrttm1Dcr Prkdcscid Tg(FCGRT)32Dcr/DcrJ negate the possibility of therapeutic mAb immunogenicity and permit efficacy testing of xenotransplants.
Potential Applications of Humanized FcRn Mice
Evaluating cancer hIgG mAbs
A major asset of hIgG mAbs as therapeutic agents is that their serum half-life is 10-20 days – about 10 times longer than other immunoglobulin isotypes.6 Their serum half-life increases in direct proportion to their affinity for FcRn, suggesting that their therapeutic efficacy would be improved by increasing this affinity. Zalevsky and his colleagues7 tested this hypothesis. They produced variants of bevacizumab (Avastin), a humanized anti-vascular endothelial growth factor (VEGF) IgG1 antibody used to treat colorectal, lung, breast, and renal cancers. They found that the hFcRn affinities of these variants (determined in vitro) are 3 to 20 times higher than those of the original Avastin. Additionally, the PKs of the Avastin variants in conventional mice fail to correlate with their affinities. In contrast, similar analyses in cDNA FCRN transgenic B6.Cg-Fcgrttm1Dcr Tg(CAG-FCGRT)276Dcr/DcrJ mice and cynomolgus monkeys demonstrate concordant and greatly extended PK profiles that correlate well with the affinity studies. Most importantly, the ability of the long-lived variants to suppress engrafted human tumors in Rag1-deficient cDNA FCRN transgenic B6.Cg-Rag1tm1Mom Fcgrttm1Dcr Tg(CAG-FCGRT)276Dcr/DcrJ mice increases significantly. These results demonstrate that humanized FcRn mice can be extremely powerful tools for the preclinical development and evaluation of hIgG mAb cancer therapies.
 ,
"...humanized FcRn mice can be applied to a wide variety of biomedical research contexts..."
 ,
Serum albumin biology and therapeutics
FcRn also regulates serum albumin homeostasis similar to the way it regulates IgG.8 Recently, Stein and her colleagues4 thoroughly compared 30 serum chemistry parameters, including albumin and albumin-dependent metabolic markers, among B6J mice, FcRn-deficient mice, and two FcRn-deficient/FCRN transgenic mouse strains. They found that FcRn-deficient mice have low IgG and serum albumin levels, which are restored to wild-type B6J levels by the FCRN transgenes. They attribute other differences among the strains to low albumin levels due to FcRn-deficiency. Similar differences occur in analbuminemic Nagase rats and hypoalbuminemic humans. Researchers can therefore use FcRn-deficient and FCRN transgenic mice to understand how FcRn controls serum albumin and to develop albumin-based therapeutics.
hFcRn expression patterns
Christianson and his colleagues9 developed a panel of mAbs directed against hFcRn. These mAbs can be used to quality-control FCRN transgene expression and investigate the expression of hFcRn in FCRN transgenic mice and humans. They also provide incisive tools for probing the biology of hFcRn.
Blocking FcRn to treat autoimmune disorders
Several studies have shown that FcRn-deficient mice are resistant to autoimmune disorders, particularly those with excessive serum concentrations of pathogenic IgG autoantibodies. The basis for this resistance can be at least partially explained by the fact that FcRn deficiency reduces the concentrations of serum IgGs, including pathogenic IgGs that promote autoimmune disease. Additionally, FcRn’s ability to affect the antigen presentation of IgG-containing immune complexes to T cells is being increasingly recognized.10 Therefore, by either or both mechanisms, FcRn may be a therapeutic target for treating autoimmune diseases.
In summary, humanized FcRn mice can be applied to a wide variety of biomedical research contexts, including preclinical development and evaluation of therapeutic mAbs and related biologics, vaccine development, autoimmune disease treatments, and albumin-based therapies.
References
1. Kuo TT, Aveson VG. 2011. Neonatal Fc receptor and IgG-based therapeutics. MAbs 3:422-30. PMID: 22048693.
2. Roopenian DC, Christianson GJ, Sproule TJ, Brown AC, Akilesh S, Jung N, Petkova S, Avanessian L, Choi EY, Shaffer DJ, Eden PA, Anderson CL. 2003. The MHC class I-like IgG receptor controls perinatal IgG transport, IgG homeostasis, and fate of IgG-Fc-coupled drugs. J Immunol 170:3528-33. PMID: 12646614.
3. Roopenian DC, Akilesh S. 2007. FcRn: the neonatal Fc receptor comes of age. Nat Rev Immunol 7:715-25. PMID: 17703228.
4. Stein C, Kling L, Proetzel G, Roopenian DC, de Angelis MH, Wolf E, Rathkolb B. 2012. Clinical chemistry of human FcRn transgenic mice. Mamm Genome 23:259-69. PMID: 22193411.
5. Petkova SB, Akilesh S, Sproule TJ, Christianson GJ, Al Khabbaz H, Brown AC, Presta LG, Meng YG, Roopenian DC. 2006. Enhanced half-life of genetically engineered human IgG1 antibodies in a humanized FcRn mouse model: potential application in humorally mediated autoimmune disease. Int Immunol 18:1759-69. PMID: 17077181.
6. Roopenian DC, Christianson GJ, Sproule TJ. 2010. Human FcRn transgenic mice for pharmacokinetic evaluation of therapeutic antibodies. Methods Mol Biol 602:93-104. PMID: 20012394.
7. Zalevsky J, Chamberlain AK, Horton HM, Karki S, Leung IWL, Sproule TJ, Lazar GA, Roopenian DC, Desjarlais JR. 2010. Enhanced antibody half-life improves in vivo activity. Nat Biotechnol 28:157-9. PMID: 20081867.
8. Andersen JT, Sandlie I. 2009. The versatile MHC class I-related FcRn protects IgG and albumin from degradation: implications for development of new diagnostics and therapeutics. Drug Metab Pharmacokinet 24:318-32. PMID: 19745559.
9. Christianson GJ, Sun VZ, Akilesh S, Pesavento E, Proetzel G, Roopenian DC. 2012. Monoclonal antibodies directed against human FcRn and their applications. MAbs 4:208-16. PMID: 22453095.
10. Baker K, Qiao SW, Kuo TT, Aveson VG, Platzer B, Andersen JT, Sandlie I, Chen Z, de Haar C, Lencer WI, Fiebiger E, Blumberg RS. 2011. Neonatal Fc receptor for IgG (FcRn) regulates cross-presentation of IgG immune complexes by CD8-CD11b+ dendritic cells. Proc Natl Acad Sci U S A 108:9927-32. PMID: 21628593.
About the author:
Ray Lambert is the technical writer for JAX® Mice &, Services at The Jackson Laboratory, Bar Harbor, ME. Humanized FcRn mice are available from The Jackson Laboratory. For more information, including purchasing, licensing, and use restrictions, see the JAX Mice Database (www.jaxmice.org) or contact JAX® Mice &, Services at 1-800-422-6423 or +1-207-288-5845.
What potential do humanized FcRn Mice have for monoclonal antibodies?