Different healthcare systems influence marketing practices by pharmaceutical companies

In this article, George Sillup and Marta Makowska compare the healthcare systems in the US, UK, Germany and Poland. They explore the different ways in which these systems influence the marketing practices of pharmaceutical companies.
Countries have different healthcare systems to provide access to medical services and prescription medications for their citizens. The healthcare system model determines access to basic care, compensation for medical professionals and how fast innovative new drugs are available from medical providers. The way healthcare systems influence the marketing practices of pharmaceutical companies is explored by comparing the healthcare systems of the United States (US), United Kingdom (UK), Germany and Poland.
US – The US Model is a combination of several government programs and private insurance programs, which have for-profit goals. They provide healthcare coverage for about 260 million Americans (about 49 million are uninsured)1. Two of the most prominent government programs are Medicare and Medicaid2, but the majority is covered by private insurers (195 million), largely through their employers (170 of 185 million)3,4. In March 2010, the Patient Protection Affordable Care Act (Obamacare) became law with the intent of providing health insurance to everyone in 20145. This change is favored by pharmaceutical companies in a pharmaceutical market that accounts for 41 percent of worldwide pharmaceutical sales. Doctors and patients are willing to choose innovative therapies; 62 percent of new drugs launched between 2007-2011 were sold in US6.
"The US Model is a combination of several government programs and private insurance programs, which have for-profit goals."
UK – The UK's healthcare delivery system is based on the Beveridge Model, which is guided by the principle that guarantees health benefits to its 63.7 million citizens7. The government designates a budget for healthcare from tax revenues for the National Health Service (NHS), which is the main employer of medical staff. It pays for healthcare services delivered to patients by medical providers. Medical expertise and preventative efforts are generally good but non-emergency cases may be subject to a long wait. Although the fifth largest pharmaceutical market in the European Union (EU), the NHS controls the majority of drug prescriptions underlying that the UK is the highest user of generic drugs and the lowest user of new drugs in Europe. 8
Germany – Germany's healthcare system is based on the Bismark Model, which has the goal of providing coverage for its 81 million citizens without an emphasis on making a profit. Since 2009, it is obligation for all citizens and long-term residents to have healthcare insurance. For the 85 percent who earn less than 49,500€ per annum, public healthcare is provided; others have the option to buy private insurance9. Insured persons contribute to their insurance and their insurer must provide access to healthcare benefits. To accomplish this, the insurers enter into an agreement with providers of medical services, e.g., doctors, hospitals, and allow patients to choose their care from providers, which signed an agreement with the insurer. A reported shortcoming of the German system is the substandard of outcomes10. As the second largest market in the EU, Germany is an important market for pharmaceutical companies but pharma marketing there has to follow strict guidelines11.
"As the second largest market in the EU, Germany is an important market for pharmaceutical companies..."
Poland – Today, the Polish healthcare system is based on Universal Health Insurance (UHI), which replaced the communist-based Siemaszko Model. After its political transformation, Poland wanted to implement the Bismark Model but, through different regulations, it is closer to the Beveridge Model. Currently, UHI is a hybrid of those models, which leads to many difficulties with healthcare delivery. Almost all 38.5 million citizens have health insurance but around three percent do not have it12. Although Poland is the sixth largest pharmaceutical market in the EU, drugs there are expensive compared to an average monthly salary of (PLN3, 600 / €850) with the majority of prescriptions being filled by generics13. Furthermore, strict pharmaceutical marketing laws prevent reps from meeting with doctors during their working hours and, then, only with permission from the physician's manager.
Important differences in pharmaceutical marketing practices
Although EU countries have different healthcare systems, there are European Directives concerning pharmaceutical marketing in member countries and rules that must be obeyed by all countries. Contrarily, the US has the same healthcare system in all its states but different marketing rules, which companies have to know. It is important to note that the core of US regulation is not federal law but guidelines that the pharmaceutical companies trade association, PhRMA, developed for member companies to follow and avoid government regulation14.
"...the US has the same healthcare system in all its states but different marketing rules..."
Direct-to-Consumer (DTC) advertising
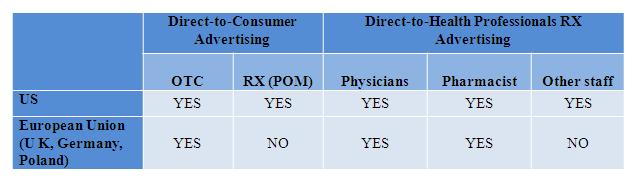
In all EU countries, DTC advertising of prescription medicines directly to the general public is not allowed. However, it is allowed in the US.
Figure 1: Comparing DTC advertising
Marketing directly to physicians
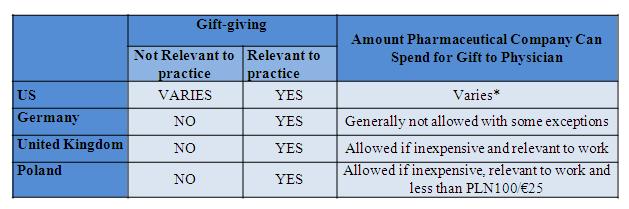
Direct marketing to physicians is one of the most important marketing approaches used by the pharmaceutical industry in all the countries. Reps armed with giveaways, such as pens and cups, meet with physicians and enjoy a high rate of success convincing physicians to prescribe their products. Previously, this was viewed as a constructive interchange between the physician and pharmaceutical company.
Figure 2: gift-giving to physicians
*$100 per PhRMA; varies by state, e.g., $25 in Vermont, <$50 in Colorado
The variation in marketing practices in the four countries suggests that different healthcare systems do influence marketing practices of pharmaceutical companies.
References
1. http://www.census.gov/popclock/ and http://www.cnn.com/2012/06/27/politics/btn-health-care
2. http://www.familiesusa.org/resources/tools-for-advocates/guides/federal-poverty-guidelines.html
3. http://www.cnn.com/2012/06/27/politics/btn-health-care
4. Many patients are covered by more than one insurance so the numbers do not total.
5. http://useconomy.about.com/od/healthcarereform/f/What-Is-Obama-Care.htm
6. http://www.efpia.eu/uploads/Figures_Key_Data_2013.pdf
7. http://www.ons.gov.uk/ons/taxonomy/index.html?nscl=Population
8. http://www.researchandmarkets.com/reports/3935/uk_pharmaceutical_industry_market_review
9. http://www.civitas.org.uk/nhs/download/germany.pdf
10. http://www.healthpowerhouse.com/files/Report-EHCI-2012.pdf
11. http://www.efpia.eu/uploads/Figures_Key_Data_2013.pdf
12. http://www.nfz.gov.pl/new/?katnr=0&dzialnr=2&artnr=4653
13. http://www.efpia.eu/uploads/Figures_Key_Data_2013.pdf
14. Sillup, Trombetta & Klimberg (2010). The 2002 PhRMA code and Pharmaceutical Marketing: Did Anybody Bother to Ask the Reps. Health Marketing Quarterly, 27:388-404.
About the author:
George P. Sillup, Ph.D., M.S.
Dr. Sillup is currently the Chairman of Pharmaceutical & Healthcare Marketing and Fellow in the Pedro Arrupe Center for business Ethics at Saint Joseph's University following 28 years of work in the diagnostic, pharmaceutical and medical device industry where he held positions from salesman to COO. During his industry tenure, Dr.Sillup has attained favorable reimbursement coverage and coding to support product launches in the U.S. and global markets and monitoring how implementation of US healthcare reform will impact healthcare delivery in the US. Dr. Sillup earned his undergraduate degree from the U.S. Military Academy and Wilkes University and his graduate degrees from Drexel University and Fielding Institute.
Marta Makowska, Ph.D., M.A.
Dr. Makowska is an adjunct at Warsaw University of Life Sciences in Poland and, as recipient of a Mobility Plus Fellowship, is a Visiting Scholar in the Pharmaceutical & Healthcare Marketing Department at Saint Joseph's University in Philadelphia. In 2004, she graduated with a Master's of Arts in Sociology and, in 2010, she finished Graduate School of Social Sciences where she defended her dissertation entitled: Ethical Standards of Pharmaceutical Marketing. A few months later she published a book by the same title, the first on this subject in Poland, and received first prize in the Federation of Financial Companies and Kozminski University Verba Veritatis contest for the best thesis in business ethics.
How do different healthcare systems influence pharma marketing practices?