A game of pharmacovigilance: from preparing dedicated teams to expanding companies’ current use of social media

Cutting Edge Information's Sarah Ray compares the drug safety methods of pharma, including how to use social media to track adverse events, with the well-known TV show, Game of Thrones.
For companies managing drug safety, the intensity of their efforts boil down to balancing internal strategies. It is possible for drug safety teams to conduct only the bare minimum pharmacovigilance activities and preserve budget and staffing resources — following only the simplest guidelines of a single market rather than creating a more exacting standard operating procedure (SOP). This narrowly focused perspective may save companies time and effort in the short term. However, it also shows a lack of foresight, which as Game of Thrones fans out there well know, is a dangerous thing — just ask Robb Stark, post-Red Wedding. The eldest Stark son failed to recognize that going back on his word would have dire consequences. This betrayal would ultimately cost him not only his life, but also those of his mother, his new bride and many of his countrymen. Taking a minimalistic approach to pharmacovigilance risks negative repercussions should a regulatory body find companies' existing drug safety protocols wanting. This approach could also prove problematic should companies encounter unanticipated drug safety challenges.
Conversely, companies with successful pharmacovigilance operations often invest great time and effort into training teams to manage drug safety responsibilities. While experiencing a resource-drain on the front end, companies that embrace more robust drug safety processes are more equipped to handle potential drug safety challenges down the road. These companies operate at a higher strategic level — not unlike Daenerys Targaryen, the Mother of Dragons — compared to their resource-conserving competitors. Through higher-level planning, this Game of Thrones heroine was able to outwit the masters at Astapor and procure a strong, trained army. Pharma may not have a host of dragons and Dothraki at their disposal, but well-developed drug safety SOPs go a long way in navigating the complex pharmacovigilance landscape.
Prioritize pharmacovigilance by developing strong employee training programs
In Game of Thrones, preparation often marks the difference between life and death, between victories and defeats. In the pharmaceutical industry, the stakes are also high. Sufficient preparation is the fine line that separates companies that receive warning letters from those awarded "best-in-class" following FDA or other governing bodies' inspections. Promoting a strong culture of pharmacovigilance requires companies to take on a number of responsibilities, from training dedicated teams to developing and managing multiple adverse event reporting channels.
In the same sense that one may not be able to shoot an arrow into the bull's-eye on the first attempt, companies do not perfect their pharmacovigilance protocols without ample training and familiarity. Consequently, drug safety training has become a priority at many of the companies. Some companies integrate pharmacovigilance training into all new employees' orientation. Others prioritize this training primarily for drug safety-facing teams — including pharmacovigilance, marketing, communication and legal teams. Training content typically explains what adverse event reports are and describes how teams should handle these incoming reports. Companies must explain their policy for incoming adverse even reports — whether employees should process the material themselves or transfer it to individuals closer to the companies' pharmacovigilance activities.
Processing adverse events is a leading responsibility for dedicated drug safety teams. Adverse events help companies document and improve upon potential safety concerns associated with their products. The frequency and nature of adverse events also prove valuable signals as companies work to develop plans set to mitigate any identified risks to patients' wellbeing.
Companies receive these adverse event reports from a number of external tools. Figure 1 shows that companies rely on reporting channels such as email correspondences, company websites and physician hotline resources. Some companies also use social media for adverse event tracking.
Establish social media as an adverse event reporting channel by expanding its existing use
Already, companies operating in the social media space — whether intended as an adverse event channel or not — have a responsibility to document and follow up with any potential adverse outcomes communicated through these forums. According to the EU's Volume 9A of The Rules Governing Medicinal Products, pharmaceutical companies are responsible for screening websites under their management for adverse events. Although the FDA has yet to release updated social media guidance, companies operating in the US take a similar approach. Because companies already show responsibility for their own online content, it follows that several surveyed companies (32%) have developed their social media resources to serve as an additional adverse event-reporting channel.
The key behind using social media as an adverse event channel stems from unlocking its potential as a value-add for companies' pharmacovigilance strategies. In Game of Thrones, Daenerys Targarygen receives three dragon eggs as wedding gifts. Beautiful and ornate, the eggs are powerless in an age where dragons are thought permanently extinct. Social media is the equivalent of a dragon egg: potentially valuable, but inert until activated.
Once hatched, the dragons bolster Daenerys' confidence in her leadership abilities and protect her from her adversaries. Alive, the dragons have become formidable weapons, supporting Daenerys' quest for the Iron Throne. In much the same way, pharmas' ability to leverage social media transforms these platforms into strategic pharmacovigilance tools.
Social media offers companies the opportunity to connect with more healthcare consumers by allowing consumers to report adverse events either directly or indirectly. Edwards and Lindquist cite one study, led by Knezevic in 2011, which created a Facebook group as an adverse event channel and tracked its effectiveness. The group found that it was able to connect with 1,000 Facebook users and receive 21 adverse event reactions within the course of seven months.
In practice, for-profit groups like PatientsLikeMe offer patients the opportunity to report adverse events directly through their website. Other companies — like Nicorette — use their Twitter accounts to connect with and encourage users. Nicorette manages a Twitter account that provides support messages, encouraging its users to continue in their efforts to quit smoking. This account also equips patients posting adverse events with the resources to fully document their outcomes.
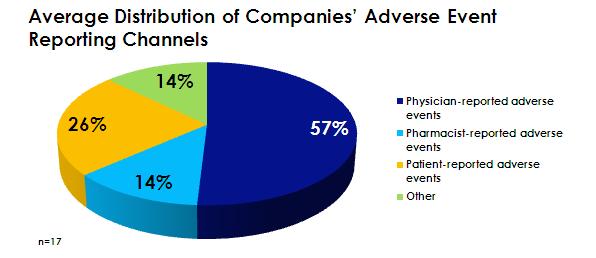
Often, consumers may not prioritize adverse event reporting to the same extent as physicians and pharmaceutical companies. As a result, not all individuals will report their adverse events experiences. Companies receive, on average, just above a quarter of adverse event reports (26%) from patients (Figure 2). Social media, however, works to increase connections between pharma and healthcare consumers. In this way, social media also helps companies address the potential for adverse events to go unreported.
Overcome social media hurdles by validating and consolidating incoming data
Data quality concerns may shape companies' approaches to social media. Implementing social media as an adverse event-reporting channel may be difficult for companies accustomed to processing uniform data collection. Adverse events received through social media may contain less information than those received through other reporting channels. In such cases, adverse events reported through social media may require additional follow-up. Consequently, companies may struggle to integrate adverse event reports received through social media with reports received from email correspondence or physician hotlines. For companies accustomed to drug safety data vetted by physicians and other healthcare professionals, verifying data received through social media may also prove challenging. To ensure the quality of data received, companies can establish safeguards against faulty adverse event reporting.
Such procedures are already in place at some social media platforms. Twitter and Facebook require that users register prior to receiving posting permissions onto user and company pages — or even accessing user pages, depending on users' privacy settings. Companies using other company-managed forums benefit from requiring individuals to register prior to being able to send or post information. Establishing this requirement helps dedicated pharmacovigilance teams verify the reported data and follow up with any additional questions or concerns surrounding the report. User registration allows companies to ensure the reporter's identity and confirm that a patient exists within each potential reporting scenario.
Often, companies — particularly those with small pharmacovigilance teams — do not have time to monitor every online resource. Moreover, not all information received through these reporting channels may be indicative of an adverse event. Companies may prioritize monitoring social media and other forums for which they are directly responsible to better manage time and financial resources. Concentrating on company-managed social media websites keeps pharmacovigilance teams' workloads manageable. Narrowing the scope of companies' social media responsibility also reduces the likelihood of companies receiving the same adverse event from different online reporting channels. In this manner, companies maintain the reliability of incoming data.
Companies may also benefit from adapting automated tools to manage incoming social media signals. Tools such as text-based or concept-based searches and data mining techniques may help companies differentiate between signals received from social media platforms and any accompanying noise. Furthermore, possible associations between adverse events and companies' products identified through tools such as Google Insights — which tracks current public concerns — may help companies prioritize among an influx of incoming social media signals.
By instituting robust pharmacovigilance training sessions, companies can better navigate existing pharmacovigilance requirements and anticipate potential challenges. Early training ensures that companies' dedicated teams recognize and address all incoming adverse event reports, particularly in newer social media channels. Leveraging the additional social media information, companies can generate more robust product safety profiles. Ultimately, by adopting a proactive drug safety stance — guarding their interests as thoroughly as the Mother of Dragons guards her children — companies achieve the greatest likelihood of remaining one step ahead of the pharmaceutical curve.
References
1. Cutting Edge Information report, "Driving Pharmacovigilance Success: Risk Management Plans and Adverse Event Reporting".
About the author:
Sarah Ray is a Research Analyst at Cutting Edge Information and newly converted fan of Game of Thrones. She graduated from NC State University with a bachelor's degree in meteorology and a minor in English. Upon joining Cutting Edge Information, Sarah has conducted benchmarking research into many pharmaceutical business functions, including pharmacovigilance, clinical development, marketing and medical affairs.
How can pharma use social media to track adverse events?











