The Cancer Vanguard: NHS and pharma partnership improves patient experience

The Pharma Challenge saw Amgen, Merck, IQVIA and uMotif collaborate with the NHS on a range of projects to improve care for cancer patients. A recent pharmaphorum and IQVIA webinar captured some of the learnings from the Cancer Vanguard-Merck-IQVIA-UMOTIF joint working initiative.
The NHS in England is in the midst of fundamental transformation and financial challenge. This is being driven by the need to re-shape the health service around an ageing population, and to create a financially sustainable healthcare system for the 21st century.
So what, if anything, can the life sciences industry do to support this process? One of the most successful examples of pharma-NHS collaboration is an initiative led by the NHS Cancer Vanguard.
As one of the cornerstones of the NHS chief executive Simon Stevens’ Five-Year Forward View, Vanguard sites have been established all over England with the aim of supercharging the move towards bold new models of care. Among the key areas identified, achieving better cancer prevention and treatment was one of the biggest challenges.
In summer 2016, the medicines optimisation team at the Cancer Vanguard launched the Pharma Challenge, an invitation for life sciences companies to be true partners in service innovation and redesigning care.
The programme goal was to help the NHS improve the effective use of its resources, enhance the understanding of the patient experience and make medicines optimisation part of routine practice.
The progress of the Pharma Challenge was put under the spotlight in a recent IQVIA/pharmaphorum roundtable discussion, “Improving outcomes through industry partnership - the Cancer Vanguard learnings”.
Project progress
Rob Duncombe, Pharmacy Director at The Christie NHS Foundation Trust and Chair of the Vanguard’s joint Medicines Optimisation Group, gave an update on the work and early outcomes from the Pharma Challenge. A total of 39 project proposals from more than 20 companies were submitted and, from these, three projects were initially selected, increasing to six projects.
“The Vanguard programme provides an unprecedented opportunity to transform care and create replicable options for the whole country,” said Duncombe. “I learned a lot in a very short period of time. One of the key elements was testing and not being afraid to fail.”
The three Cancer Vanguard hospital trusts – The Christie NHS Foundation Trust, the Royal Marsden NHS Foundation Trust and University College London Hospitals NHS Foundation Trust (UCLH) – spend more than £120 million on cancer medicines annually, according to Duncombe, while year-on-year expenditure is increasing by about 8%.
“I felt there was a real opportunity to look at the management and optimisation of the use of medicines to deliver better outcomes for patients, with better use of resources.”
Since then, the Vanguard has gone live with four of the six projects.
- The UCLH Cancer Collaborative partnered with Amgen to map out the most efficient out-of-hospital administration of denosumab, a therapy for secondary breast cancer in the bone. The project has developed an ‘options appraisal’ document and user guide toolkit, aimed at helping NHS trusts and clinical commissioning groups (CCGs) to adopt the model.
- Sandoz proposed an education and engagement programme with healthcare professionals across the Cancer Vanguard about the use of biosimilar medicines. These can help the NHS make significant savings, but uptake can be hindered by doubts about the medicines among nurses, doctors and patients.
- Celgene worked with the Vanguard to develop an ‘Interactive Medicines Optimisation and Compliance Dashboard and Evaluation Framework’ using the Systemic Anti-Cancer Therapy dataset.
- IQVIA, a human data science company brought about by the merger of Quintiles and IMS Health, worked in partnership with Merck, uMotif and The Christie, a specialist cancer centre in Manchester, to analyse medicine usage data and quantify costs associated with unwarranted variation.
Mapping cancer care
The Christie collaboration looked at how to use NHS resources more efficiently, improving quality, reducing unwarranted variation in care and establishing a better understanding of the experience of cancer patients during treatment.
Stephen Jowett, Country Lead for Health System Engagement, IQVIA, explained: “This partnership working across the health system focused on the total service, not just the administration of the medicine. It used leading-edge data analytics to analyse, benchmark and visualise the current state of the cancer pathways within the Cancer Vanguard Trusts.”
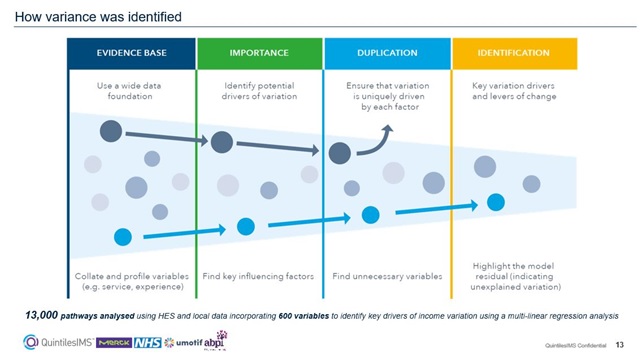
The project also looked at how to improve patient outcomes and experiences, the flow of treatment and the usage of medicine. By collecting relevant data, it has helped to shape an evidence-based and patient-centric approach to service redesign and resource optimisation.
One novel aspect of the study involved asking patients to record their symptoms – such as mobility, and energy levels – using uMotif’s smartphone-based app.
Stuart Hill, Head of Marketing – Oncology, UK & Ireland, Merck said the partnership allowed medicines usage to be analysed and patient pathways to be mapped. This helped to create a picture of the patient experience throughout the patient journey.
“First, we wanted to understand medication variance in oncology drugs used to treat the most common cancers. Then we could analyse the drivers of this variation in the service line. It was a fantastic opportunity to highlight potential variance from NICE therapy guidelines in a collaborative way.”
The research found a sevenfold variance in the proportional use of biological therapy versus chemotherapy between the peer comparators. This allowed the different NHS trusts to compare and contrast their total service to patients, and see where they could improve and learn from each other.
Stephen commented: “It was phenomenally successful in that we collected 111,000 data points over a 35-week project. One patient entered their symptoms on 188 days over a 200-day period, which was quite incredible.
“The clinical team at The Christie are now keen to start this as a randomised clinical trial, to see how their treatment processes can be enhanced using these kinds of technologies to remotely track symptoms over time.”
Spread the word – and best practice
The Pharma Challenge is seen as a success by all sides who took part, but now the test is to ensure its learnings are diffused throughout the NHS. The ABPI’s NHS Engagement Partner (London) and Specialised Commissioning Lead, Mike Ringe, stated: “How do we spread learnings from the Pharma Challenge? We want to build momentum and make sure the project isn’t just a one-off.”
According to a poll of the webinar’s largely pharma industry audience, 45% hadn’t heard of the initiative before. The need to educate all stakeholders and spread the message of the Pharma Challenge’s success is therefore clearly necessary. Mike Ringe said that the partnership demonstrated how sticking to sound principles of collaboration can pay off. These include having clear expectations, shared organisational commitment, and clear clinical benefits. Above all, project insights need to be translated into real change in order to deliver tangible results to patients.
Stephen Jowett added: “It’s important that the energy, drive and determination that contributors have is translated into reality. That requires people to have the autonomy to drive these projects through to completion.”
He concluded that it was always great to try something out without fear of failure, but collaborations needed to have focus to deliver on these concepts.
“These projects always need clinician buy-in and full patient engagement to ensure a study eventually becomes a real clinical pathway which can then be scaled up.”
View the webinar here: Improving Outcomes Through Industry Partnership – Learnings from the Cancer Vanguard.












