News
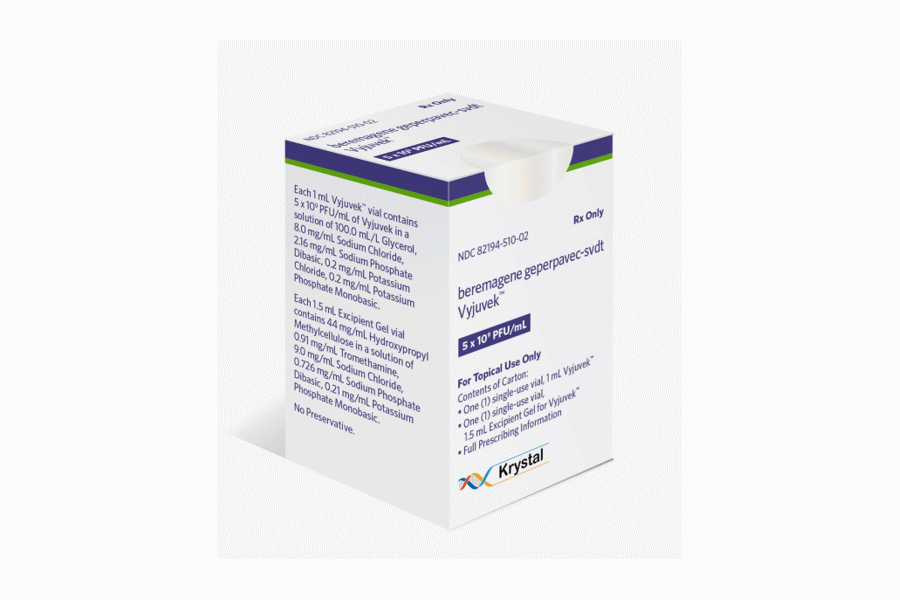
FDA clears Vyjuvek as first topical gene therapy
The FDA has approved Krystal Biotech’s gene therapy Vyjuvek for dystrophic epidermolysis bullosa (DEB), a rare genetic disease characterised by fragile skin that can split



