Solution Spotlight: Viseven’s eWizard

With the right process and tools, localisation can become a well-oiled machine. Our approach involving eWizard has been tried and tested by multiple life sciences businesses, proving its efficiency.
What problem or status quo deficiency are you solving?
For pharma companies expanding into new markets – or those already there – localisation remains a persistent challenge. Even with the most efficient translation partners and software, the process can feel like email ping-pong. Managing multiple versions, approvals, and last-minute edits manually becomes overwhelming, especially when scaling international content delivery. As a result, your campaigns take longer to deploy.
Yet, if you want to truly resonate with local audiences, good localisation is in no way optional. It’s the key to maintaining brand integrity, ensuring regulatory compliance, and driving meaningful engagement. And without a seamless, efficient process, even the most carefully crafted global content can lose its impact.
Localisation in life sciences is more than a checkbox because if your content doesn’t speak the local language (literally and culturally) it’s just noise. Whether it’s regulatory documents, instructions for use, package labelling, or marketing materials, getting it right means the difference between engagement and irrelevance.
What has made this problem challenging to address in the past?
We don’t need to tell you how frustrating localisation can get – you’ve likely dealt with many of these challenges firsthand. Missed deadlines, ballooning costs, endless back-and-forths. We’ve seen this time and time again while helping life sciences companies clean up their localisation workflows.
More and more life sciences companies are putting their money where their markets are. High-quality localisation isn’t just a cost of doing business – it’s a strategic investment. The reason is because it’s crucial for regulatory compliance, patient safety, and, ultimately, business growth.
And the numbers back it up. According to Grand View Research, the global life sciences translation services market hit $1.45 billion in 2023 and is set to grow at a CAGR of 8.43% through 2030.
Pharma companies are doubling down on accurate, culturally adapted content to strengthen their position in global markets. Cutting-edge translation tech, rising demand for localised healthcare communications, and the need for culturally relevant marketing are fuelling this growth.
It’s not just regulatory bodies and pharma marketers pushing for better localisation – customers demand it too. The Can’t Read, Won’t Buy report, which surveyed 8,709 consumers across 29 countries, found that 65% of respondents prefer content in their native language, while 40% would not purchase a product or service if it wasn’t available in their language.
This preference directly impacts revenue. A staggering 84% of marketers report that localisation has had a moderately to extremely positive impact on revenue growth. The connection is clear: if customers can engage with content in their own language, they’re far more likely to trust the brand and convert.
Yes, localisation helps drive sales, but that’s just the start. It’s also a powerful tool for customer experience, retention, and brand awareness.
If content personalisation were a house, localisation would be the foundation. Today’s pharma marketing is all about delivering the right message to the right audience at the right time — and that starts with language.
Localisation enables companies to form deeper connections with local healthcare providers and patients by offering content that is not only relevant, but also culturally aligned. This connection strengthens the brand’s reputation, demonstrating that the company understands and respects the unique needs of each market, rather than applying a one-size-fits-all approach.
It’s not just about perception either. By ensuring that healthcare information is accurate, clear, and compliant with local regulations, companies reduce the chances of miscommunication and build trust with both patients and healthcare professionals.
In the end, the benefits extend far beyond immediate revenue, solidifying the brand’s position in competitive markets while fostering loyalty and long-term engagement.
Even with advancements in automation, many pharma companies still rely on manual processes for translating and adapting content. This not only drives up costs, but also opens the door to more errors. And here’s the kicker – only 28% of pharma companies say they can repurpose content without a lot of manual work.
Reusability and modular content are a whole other conversation when it comes to local markets. Local teams create content from scratch (up to 80%), instead of reusing or adapting global assets. Same with modularisation: many local markets aren’t equipped with the right tools or expertise to make modular content work.
Finally, the lack of comprehensive instruments shows when teams try to coordinate the work across different time zones or find the necessary final-final version
What new innovations does your solution bring to bear on these challenges?
But here’s the good news: you can surmount these obstacles. Once you know what’s causing the bottlenecks, you can take steps to fix them. Let’s walk through the most common issues that derail localisation and explore the impact they have on your business.
With the right process and tools, localisation can become a well-oiled machine. Our approach involving eWizard has been tried and tested by multiple life sciences businesses, proving its efficiency.
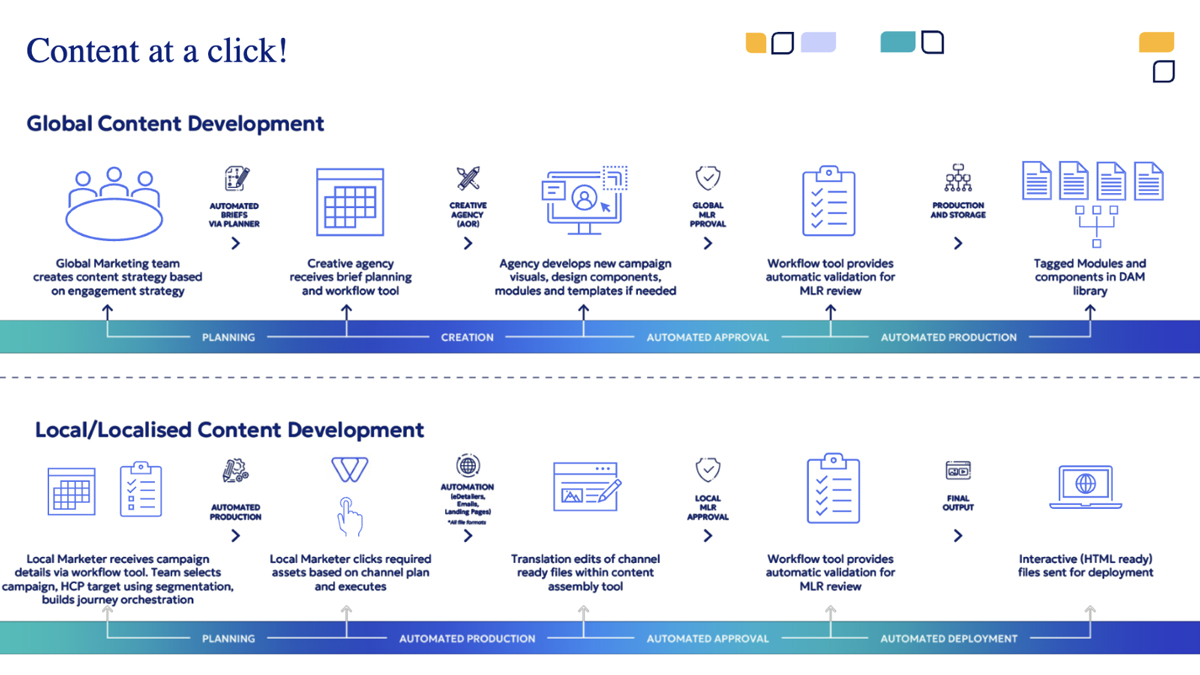
How does your solution work?
Step 1: Campaign briefing
Localisation begins with a detailed campaign brief given through eWizard. This brief includes everything local teams need to get started — style guides, glossaries, and market-specific instructions — all stored in the centralised repository. By automating brief distribution, the platform reduces the time spent on back-and-forth coordination and ensures all teams have immediate access to the latest materials.
Step 2: Content review
Local teams review the content to ensure clarity and accuracy. If any issues arise, they can flag the content through eWizard’s collaboration tools, prompting revisions by the global team. This prevents errors from trickling into the localised versions, saving time and rework later.
Step 3: Localisation kick-off
Once the materials are in good shape, local teams select what they need based on their channel plan. Luckily, they don’t need to start from scratch. With the recent introduction of Modular Content 2.0 to eWizard, it’s quite simple for local teams to adapt pre-approved content blocks, saving both time and effort while keeping the messaging on-brand.
Step 4: Tailoring translation and visuals
Next, the platform’s auto-translation feature steps in to handle the initial translation, speeding up what could otherwise take much longer. But it’s not a “set it and forget it” system — subject-matter experts and medical professionals review and tweak the text to make sure it’s both accurate and culturally appropriate. This mix of automation and expertise strikes the perfect balance between speed and quality.
Language isn’t the only thing that needs localising. Visuals also play a big role in connecting with audiences. Our design team customises images and other visuals to fit cultural expectations and norms. The files are accessed and uploaded via the centralised asset library to avoid unnecessary duplication (and not to waste time).
Step 5: Checks and approval
Compliance is crucial, but it shouldn’t slow you down. Our MLR acceleration engine scans the translated content to catch any errors or inconsistencies before it goes for final approval. This helps prevent back-and-forth delays during the review process.
Step 6: Content ready for deployment
After approval, HTML-ready files are generated and sent out for deployment. eWizard ensures that the localised content is optimised for the specific channels where it will appear, whether it’s an email, website, e-Detailer, or social media post.
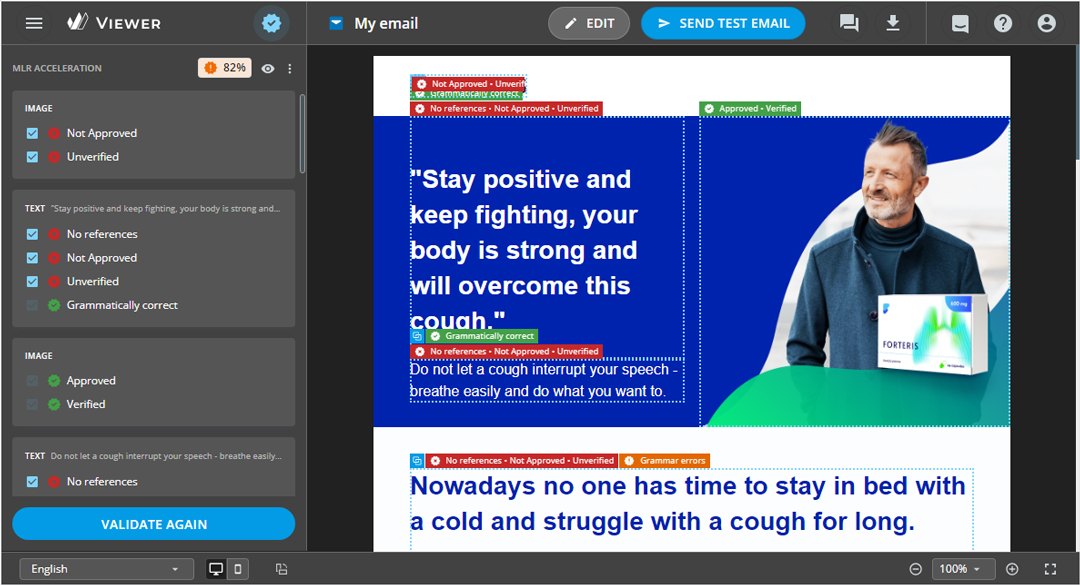
How does your solution improve the experience for the end user?
Now or in the future, pharma content localisation shouldn’t be about choosing between speed or quality. You can get both through smart processes and the right technology.
With our proposed set-up, life sciences companies can tackle localisation with confidence, knowing they’re saving time, cutting costs, and delivering content that hits the mark in every market.
Companies that invest in optimising their localisation processes today are not just solving an immediate operational challenge — they're building the base for successful global engagement in the years to come. So, embrace the modern solutions that combine automation with human expertise, and you can be confident that your message resonates authentically with the people from any market.
About Viseven

Viseven is a global MarTech company specializing in digital content solutions for the Life Sciences and Pharma industries. With over 15 years of expertise, Viseven empowers pharmaceutical companies and their production agencies with AI-driven content management and automation solutions.
Our flagship eWizard Platform streamlines content planning, creation, distribution, and management—enhancing efficiency, reducing operational costs, and accelerating brand time-to-market. Designed for omnichannel and multichannel engagement, eWizard optimizes campaign management, data collection, and performance analysis, ensuring continuous message improvement for Brand Managers and Content Operations teams.
Visit us at viseven.com or follow us on social media: LinkedIn











