Gene therapies for prevalent diseases in Europe – the perfect storm of economic sustainability?

In this article, Research Partnership reviews why the path to commercialisation for the half a dozen approvals in Europe over the last few years has not been smooth, and shares feedback gathered from our payer network on the outlook for gene therapy market access in Europe, especially considering the shift to more prevalent diseases.
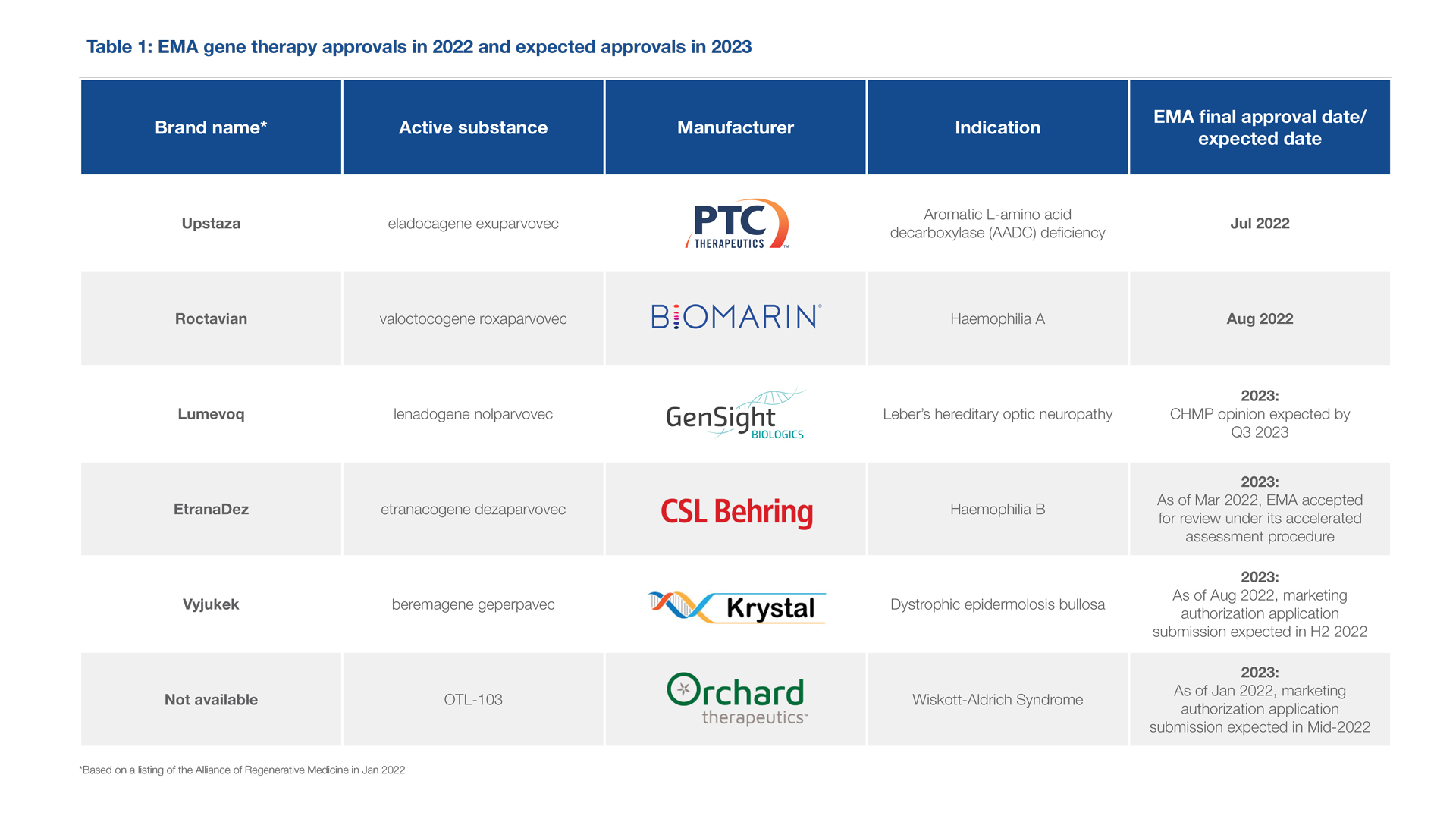
In January, the Alliance for Regenerative Medicine (ARM)’s Cell and Gene State of the Industry briefing earmarked 2022 as a record year for approval of gene therapies for rare diseases. This has not transpired. As of September, only three gene therapies from the ARM list have received European Medicine Agency (EMA) approval so far, with three expected to reach the market in 2023 (see Table 1).
Developing, manufacturing, and getting a gene therapy to a patient is complex and expensive. Gene therapies are associated with a high cost of goods and require a price reflective of their curative value. For ultra-orphan and orphan diseases, the small numbers of patients who will benefit mean high prices are needed to ensure profitability and these have proven challenging to achieve.
While the industry remains active in developing advanced therapies (including gene therapies) within the rare disease space, including haematological disorders and eye disorders, there is an increasing shift from the advanced therapy sector as a whole to more prevalent diseases (see Table 2).
• Read the full article in pharmaphorum's Deep Dive digital magazine












