Could the way you work be inhibiting digital success?

Pharma companies are investing a lot of money in digital products, but not all of them are delivering the expected results.
The assumption is often that the initial concept was flawed, with brands abandoning unsuccessful products to focus on the next big thing, throwing significant investment of both time and money down the drain, when simple changes made before, during, and after launch could make the world of difference.
With ways of working ultimately underpinning success, there are three key questions that can help you to identify areas that could be inhibiting digital product success.
1. How are you ensuring what you are delivering is meeting your end users’ needs?
Data collection is critical for helping to define both the needs of the products and your next steps in its creation.
While research and analytics are great tools to validate an existing product, it can be even more valuable when used from the very start of the design and development process. Validating the need for the product, as well as uncovering features that would be the most valuable to end users before you even begin, can help to save a lot of time and money.
Once you have a product you think is ready to launch, it is important to test it with the intended audience to confirm that it is meeting their needs, reducing the resources that will be needed to update the product later if it falls flat. This is one of the most important phases of the entire product development process, but it often meets with resistance from leaders who are focused on delivering a product by a specific date or timeline. While it could lead to a one- to three-month delay if changes are required, releasing the ‘right’ product to the market is more likely to result in engaged and returning customers.
The job does not end with the product launch, as changing behaviours and needs often require adjustments to be made later on. Measuring and repeatedly validating your product will ensure you’re prioritising the correct areas for improvement. You may have an excellent product that requires a login, but if users are regularly dropping off during the registration stage there is very little value to the product you have created. The drop off itself only provides limited information, but carrying out moderated and unmoderated research to gather feedback from real users can give you a much better understanding of why your current product may not be working for them, and provide a clear roadmap for improvement.
The continuous measurement and improvement mindset needs to become integral to the way your team works in order to create products that regularly succeed both at and after launch. Once research and validation becomes part of your cycle of development, it begins to have minimal impact on providing product updates.
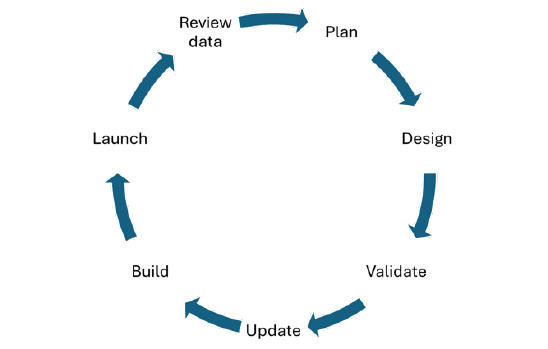
This may come with some stakeholder pushback initially, so it is crucial to bring them along for the journey. By keeping them engaged, and ensuring that they understand both the target audience and the value of implementing certain changes, it can help the entire process run more smoothly.
2. Is your process and team able to adapt to change?
One of the biggest downfalls when it comes to developing and managing digital pharma and healthcare products is the inability to adapt to change. Change can come in many forms, including shifting priorities within the organisation, environmental changes, such as the impact of the pandemic, or technology changes, like native software updates that can fundamentally change requirements.
The long-standing waterfall approach to development may be efficient, but offers very little room for change once the process has begun. Not only can this result in a product being outdated by the time it comes to launch, wasting both time and money, but where major changes are needed it can often mean teams have to go back to the drawing board altogether.
This is one of the reasons we have seen such high adoption of agile working over the past 15 years, as it allows greater flexibility throughout the design and development process. It allows for faster decision making, and gives teams the ability to tackle problems as they arise and find solutions more effectively.
3. Is the team structured to get the best out of everyone?
Having the right people in the right roles and a structure that allows everyone to work together smoothly facilitates successful digital product development. Alignment between stakeholders, product owners, scrum masters, and project managers is critical for developing the right product at the right pace.
If you are using an agile working style, ensure that the sprint structure you have in place supports the team. Make sure that you are building in enough time for retrospective meetings after a period of work is complete, regular reviews, and team discussions, all of which help to keep the project on track.
If you are facing time pressures, it is important to remember that adding more resources for a team may help to develop the product more quickly, but that it can negatively impact quality and increase the likelihood of team burnout. If it is likely that team growth will be needed in the future, ensure there is a plan in place from the beginning that can be activated as required.
Digital product success hinges on the processes in place that help it get to a point where it is fit-for-purpose at launch and allows it to continue to evolve with the end users’ needs and changes in the wider environment.
It is critical that pharma companies approach digital product design with an adaptable mindset, and have regular checkpoints that allows validation of the current direction and the flexibility to change path should it be necessary. Your ways of working should reflect the fact that the product needs to be fluid both during and after the development process, in order to deliver value for the end users and ensure that the investment delivers the desired return.











