The power of data science platforms to cut through the complexity of modern-day clinical research

Biopharmaceutical companies across the globe are rapidly expanding their pipelines as they aim to break new ground in different disease areas, on behalf of patients awaiting life-changing treatments. These breakthroughs, however, are taking place amid growing pressures facing clinicians and the wider pharmaceutical industry.
With insights into disease pathways growing in complexity, so too are clinical trials becoming more complex, which can lengthen trial timelines and increase costs. In addition, companies are often required to manage and deliver multiple clinical trials simultaneously, as opposed to focusing on keeping a single trial on time and on budget.
To meet these pressures, clinicians must be able to access high-quality trial data in real-time, collaborate with colleagues across all trial sites, and use advanced analytics and visualisation tools to make informed decisions. This is where a fast and flexible data and analytics platform is a vital tool to support the modern clinical research landscape, as drug development becomes increasingly reliant on vast and varied data sources.
A data-driven platform can provide centralised data management, advanced analytical capabilities, data visualisation, risk-based monitoring, and collaboration to increase efficiency in the drug development process and ultimately improve the quality, accuracy, and utility of clinical data. Such a platform can support clinicians in all phases of drug development, from early-phase exploratory studies to late-phase pivotal trials.
Matching accuracy with efficiency for faster decision-making
A clinical data science platform provides scientists with a centralised repository with data management capabilities, helping to streamline the multifaceted processes of data ingestion, unification, standardisation, mapping, and harmonisation.
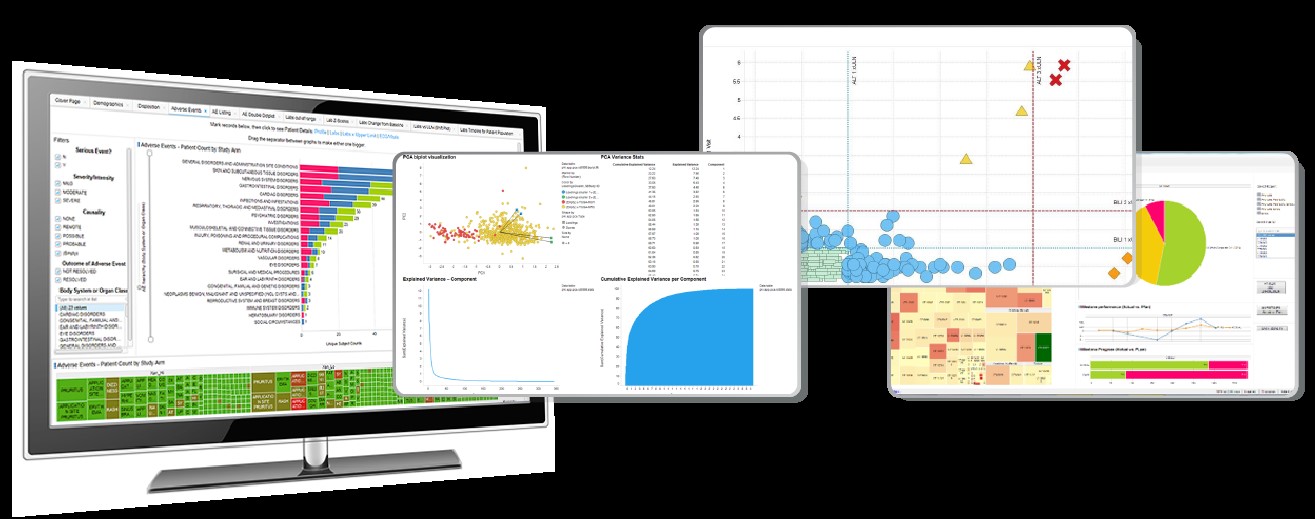
Through the platform, data from various sources – such as electronic health records (EHRs), wearable devices, lab results, and more – can be stored, accessed, and analysed in real-time. Clinicians can drill down into data at an aggregate population level, such as per site, per demographics, or per treatment cohort, enabling a more detailed and tailored view of results. Clinicians can further drill down to the patient level to provide an even more granular view into patient safety and drug efficacy.
The platform can also provide data managers and clinical monitors with automated alerts to inform ongoing monitoring of data quality. Data management across this centralised repository promotes uniformity, reduces discrepancies, and ensures that the collected data is of high quality, accuracy, and reliability, supporting clinicians and data scientists to make more effective analyses, predictions, and decisions.
A data platform provides advanced analytical capabilities by using predictive analytics and machine learning to predict trends, model disease progression, give insights into patient behaviour and drug efficacy, and identify potential drug interactions or side effects. These advanced analytics can be provided in real-time, helping clinicians make informed choices at every stage of drug development, and can be processed swiftly in high volumes, significantly reducing the time from drug conceptualisation to market launch. Furthermore, predictive analytics can help in the early identification of potential drug failures or risks, allowing organisations to allocate their resources to the more promising drug candidates and to save substantial funds and resources that might have been expended on drugs that will ultimately fail.
Visualisation tools within a data platform can transform complex data sets into understandable and actionable insights. The clinical data has been harmonised and is F.A.I.R. – Findable, Accessible, Interoperable, and Reusable – data that streamlines the development and reuse of analytics. Graphs, heat maps, and other visual aids help clinicians quickly grasp the significance of their data, aiding in swifter decision-making processes.
Clinical data science platforms can provide risk-based monitoring and predictive analysis by utilising algorithms to foresee potential risks in clinical trials. By highlighting areas of concern in real-time, scientists can intervene promptly to ensure potential adverse effects or other issues are addressed, enhancing the safety of participants involved in trials.
A data platform improves collaboration across clinical trials and therapeutic areas. Cross-study and within study search tools allow clinical trial information to be aggregated and parsed across trial sites. Providing a common set of analytics across functional groups also improves cross-functional collaboration and operational efficiencies by reducing reliance on biostatisticians to fulfil reporting requests. This frees biostatisticians and others for higher value projects and eliminates delays typically involved in reporting.
Additionally, modern platforms are designed for enhanced interoperability due to their compatibility with various data formats and standards. This ensures seamless integration with other systems, allowing organisations to introduce the latest innovations in technologies and methodologies into their processes, and promotes collaborative research across organisations and disciplines.
Unlocking the potential of data science platforms
Data science platforms have begun to transform the delivery of analytics in clinical research. Revvity Signals Clinical, for example, provides a SaaS, cloud-native, end-to-end clinical data science platform to bring together all clinical development data into one location and presents rapid actionable insights to drive decisions that are relevant to roles, studies, and therapies. This streamlines clinical data workflows and reduces the time spent on manual analytic data preparation tasks.
Such platforms allow faster delivery of the real-time analytics and visualisations that clinicians need to inform critical study decisions and keep the trial on track, delivering comprehensive data management capabilities, and ensuring efficient standardisation and harmonisation of clinical trial data. The net result is a single point of access for analysis-ready clinical data.
The future of data-driven drug development
In today's data-driven clinical development environment, a clinical data science platform is not just a luxury but a necessity. Such a platform can provide robust data management and advanced analytics to help overcome obstacles to data-driven decisions in clinical development, enabling clinicians to integrate the F.A.I.R. principles into drug development by making clinical data more Findable, Accessible, Interoperable, and Reusable, and thereby enhancing the quality and utility of data, which makes the data a more valuable asset in drug development. It streamlines operations, enhances decision-making, and positions organisations to lead in the race to develop ground-breaking treatments that can help patients and improve human health.
About the authors
 Brent Meyers currently leads Revvity Signals's Clinical business team, directing product management, marketing, and business development. He has been building analytics solutions and leading technical delivery teams for 20 years in the clinical trial and defence industries. Meyers holds a BA from The Citadel and an MBA from Meredith College.
Brent Meyers currently leads Revvity Signals's Clinical business team, directing product management, marketing, and business development. He has been building analytics solutions and leading technical delivery teams for 20 years in the clinical trial and defence industries. Meyers holds a BA from The Citadel and an MBA from Meredith College.
 Philip Ross seeks to partner with healthcare and pharmaceutical partners in enriching Revvity Signals's understanding of novel treatments. Formally a practitioner with Pfizer and Bristol-Myers Squibb, Ross brings deep pharmaceutical experience in the visualisation of clinical data to drive clinical decision-making. He has a PhD in Pharmacology from Ohio State University.
Philip Ross seeks to partner with healthcare and pharmaceutical partners in enriching Revvity Signals's understanding of novel treatments. Formally a practitioner with Pfizer and Bristol-Myers Squibb, Ross brings deep pharmaceutical experience in the visualisation of clinical data to drive clinical decision-making. He has a PhD in Pharmacology from Ohio State University.












