The longitudinal history of medicines innovation: Part One
As we move forwards in public discussions on pharmaceutical innovation and its value to patients and society, it is helpful to ground conversations in a solid understanding of how medicines development occurs.
CRA’s Life Sciences Practice team along with experts at Johnson & Johnson recently conducted a landscape review of how complex pharmaceutical innovation happens in practice, leveraging three therapy area case studies: HIV/AIDS, multiple sclerosis, and multiple myeloma. This article is the first of two parts.
Innovative medicines development occurs in a healthy ecosystem
Much has been written on the traditional drug discovery and development process in the biopharmaceutical industry, but few studies have put specific innovations within the context of the successive steps forwards in our scientific understanding, a dynamic process full of false starts and competing lines of investigation, and the roles of public and private institutions.
As Figure 1 illustrates, there is a distinction between private and public sector roles within different components of the biopharmaceutical innovation process. Phased investments in R&D occur through the roles and investments of these key players, resulting in benefits to patients, caregivers, health systems, and society throughout the post-approval access of new medicines. Within the high-risk investment phase, there are often innovation failures that result in loss of value of the investment made and future returns for private sector companies. The activities during this high-risk, often iterative phase of innovation lead to the second half of the pathway, during which patient outcomes are improved and health system benefits are generated.
Figure 1: Private and Public Roles in the Biopharmaceutical Innovation Process
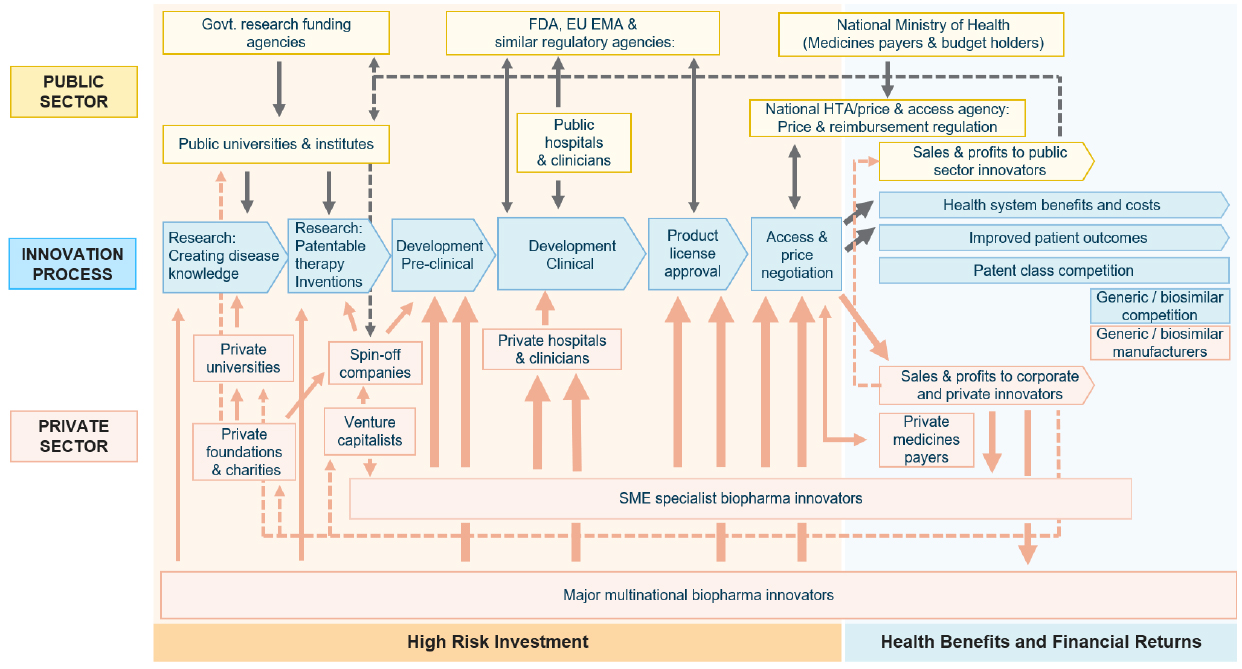
Source: CRA (2023)
Looking at three therapeutic areas: HIV/AIDS, multiple sclerosis (MS), and multiple myeloma (MM), we explore the origins of different classes of medicines and connect the roles of public and private key players in the research phase, clinical development, and integration of innovations into healthcare systems.
Translating early basic research into new treatments – the role of biopharmaceutical companies
In all three therapy areas, while the initial scientific discoveries were often made in academic research, it was through partnerships and capabilities contributed by biopharmaceutical companies that these insights were ultimately translated into large-scale clinical programmes and new treatments.
Innovation in HIV/AIDS relied heavily on academic research into the molecular biology of HIV’s lifecycle, transmission, and manifestation of disease, and on companies’ screening internal libraries to identify compounds with activity against the virus. Building on basic research in the role of HIV protease, by the late 1980s, scientists at research institutes and pharmaceutical companies – including the US National Cancer Institute (NCI); Birkbeck, University of London; and a group at Merck, Sharp and Dohme (MSD) – began working to solve the crystal structure of the viral protein.1 The discovery that HIV protease was a member of the aspartic protease family was a key discovery and led to many companies working to develop and test various compounds. In 1995, the first drug was approved for use in HIV and a number of other protease inhibitors followed.
In MS, the importance of multiple entities working in a disease area would prove pivotal in the development of the first approved treatment, interferon β-1b for relapsing remitting MS (RRMS) in 1993. First, scientists established evidence that interferons had immuno-modulatory properties, research originally targeted as anti-cancer and anti-viral, with anti-viral research in particular supported by companies including Glaxo Laboratories, ICI Pharmaceuticals, and Burroughs Wellcome.2 Second, scientists investigating MS pathogenesis confirmed that the natural history of MS could be altered with an immunomodulating approach, generating a rationale for the clinical use of interferons in the treatment of MS. Biopharmaceutical companies learned how to produce interferons at scale, enabling the clinical trials needed to bring interferons to patients.3
For MM, as scientists better understood the role of the proteasome pathway and cell growth and division, and thus its potential for anti-tumour effects, the development of proteasome inhibitors also advanced. It was biotech originating from academia that led to this first novel class of treatments in decades, the first proteasome inhibitor bortezomib, which was followed by the second- and third-in-class treatments, carfilzomib and ixazomib, respectively. The engineering of large molecule and cell therapies also began in academia, while partnerships between smaller companies and larger, more established companies drove the development of CAR-T and mAb therapies specific to MM.
Innovation is a cycle of development – new therapies build on advances in scientific understanding and lived experience with approved therapies
Understanding of our case study diseases has improved over time, providing insight into the mechanisms of action of existing treatments and directing innovation towards new compounds and targets. This cycle of development also builds on learnings from the use of approved therapies, enabling the development of superior treatments better targeted for individual patients.
For example, the earliest attempts to understand HIV depended on understanding its molecular biology, with a focus on solving and publishing the crystal structure of the HIV protease, allowing targeting by several new therapies from biopharmaceutical companies: first AZT (azidothymidine or zidovudine) and other the nucleoside reverse transcriptase inhibitors (NRTIs), then protease inhibitors and non-nucleoside reverse transcriptase inhibitors (NNRTIs). Once protease inhibitors reached the market, patient challenges around dosing and adherence remained, due to complex combination regimens.4 Recently, innovation has continued to build on the success of combination therapies by focusing on optimising the dose regimens to improve patient adherence and effectiveness in the long term.
In MS, scientists continue to gain a better understanding of MS pathophysiology. Research on the role of B-cells has led biopharmaceutical companies to several new treatments, including B-cell targeting mAbs, such as ocrelizumab and ofatumumab. This shift to a B-cell mediated conception of MS pathophysiology has been a key development in disease understanding since the 1990s.
References
- Sansom, C. (2009). Molecules made to measure. Chemistry World. Retrieved from https://www.rsc.org/images/Drug%20design%20HIV_tcm18-166406.pdf. Accessed October 13, 2021.
- Taylor, M. W. (2014) Viruses and Man: A History of Interactions. Springer International Publishing.
- Murray, T. J. (2004). Multiple sclerosis: the history of a disease. Demos Medical Publishing.
- National Institute of Allergy and Infectious Diseases. National Institutes of Health Website. Antiretroviral Drug Discovery and Development. Available at: https://www.niaid.nih.gov/diseases-conditions/antiretroviral-drug-development. Accessed October 12, 2021.
About the authors
 Tim Wilsdon is VP in the life sciences practice at CRA and leads the policy sector in the global life sciences practice. He is a regular contributor to international conferences on health policy and has also acted as an expert in international arbitrations involving multinational pharmaceutical companies. He was responsible for leading an assignment for the European Commission, determining whether there was a global crisis in innovation and the risks of importation.
Tim Wilsdon is VP in the life sciences practice at CRA and leads the policy sector in the global life sciences practice. He is a regular contributor to international conferences on health policy and has also acted as an expert in international arbitrations involving multinational pharmaceutical companies. He was responsible for leading an assignment for the European Commission, determining whether there was a global crisis in innovation and the risks of importation.
 Clara Zacharko is a consulting associate in the life sciences practice at CRA. She focuses on health policy consulting and has experience in analysing and understanding the impacts of changes in the policy landscape on pharmaceutical innovation in North American and EU markets.
Clara Zacharko is a consulting associate in the life sciences practice at CRA. She focuses on health policy consulting and has experience in analysing and understanding the impacts of changes in the policy landscape on pharmaceutical innovation in North American and EU markets.
 Hugh Nicholl is an associate in the life sciences practice at CRA. He works across strategy and policy consulting, with a focus on the health policy landscape and market access for pharmaceuticals in the EU.
Hugh Nicholl is an associate in the life sciences practice at CRA. He works across strategy and policy consulting, with a focus on the health policy landscape and market access for pharmaceuticals in the EU.
The authors wish to acknowledge the contributions of Dr Reina Benabou, Dr Richard E. Nettles, Dr J. Blake Bartlett, and Danielle Rollmann from Janssen, and Adrian Griffin from Johnson & Johnson to this article. The research for the underlying case studies was funded by The Janssen Pharmaceutical Companies of Johnson & Johnson.
The views expressed herein are the authors’ and not those of Charles River Associates (CRA) or any of the organisations with which the authors are affiliated.











