The ryze-elluminate integration to maximise automation in clinical trials

The integration of a Clinical Metadata Repository (CMDR) and a Clinical Data Repository (CDR) presents the opportunity to maximize automation across the end-to-end clinical trial lifecycle. It also affords the ability to leverage wider benefits that can’t be achieved through just one solution.
But there’s no use in technology partnerships without tangible interoperability in place. There must be a live integration that’s ready for clients to use from day one. You must be able to demonstrate the value of multi-vendor technology, and show how a joined-up solution can be leveraged to solve problems, speed up processes, and improve quality. And that means investing in true interoperability between platforms, in order to provide maximum capabilities and deliver against client objectives.
Technology partnership
Over two years ago Formedix joined forces with eClinical Solutions, a leading provider of solutions for collecting, standardizing and reporting clinical research data. Their elluminate platform allows life science companies to integrate and unify their data sources – including EDC, eCOA and labs – for streamlined data review and data insights.
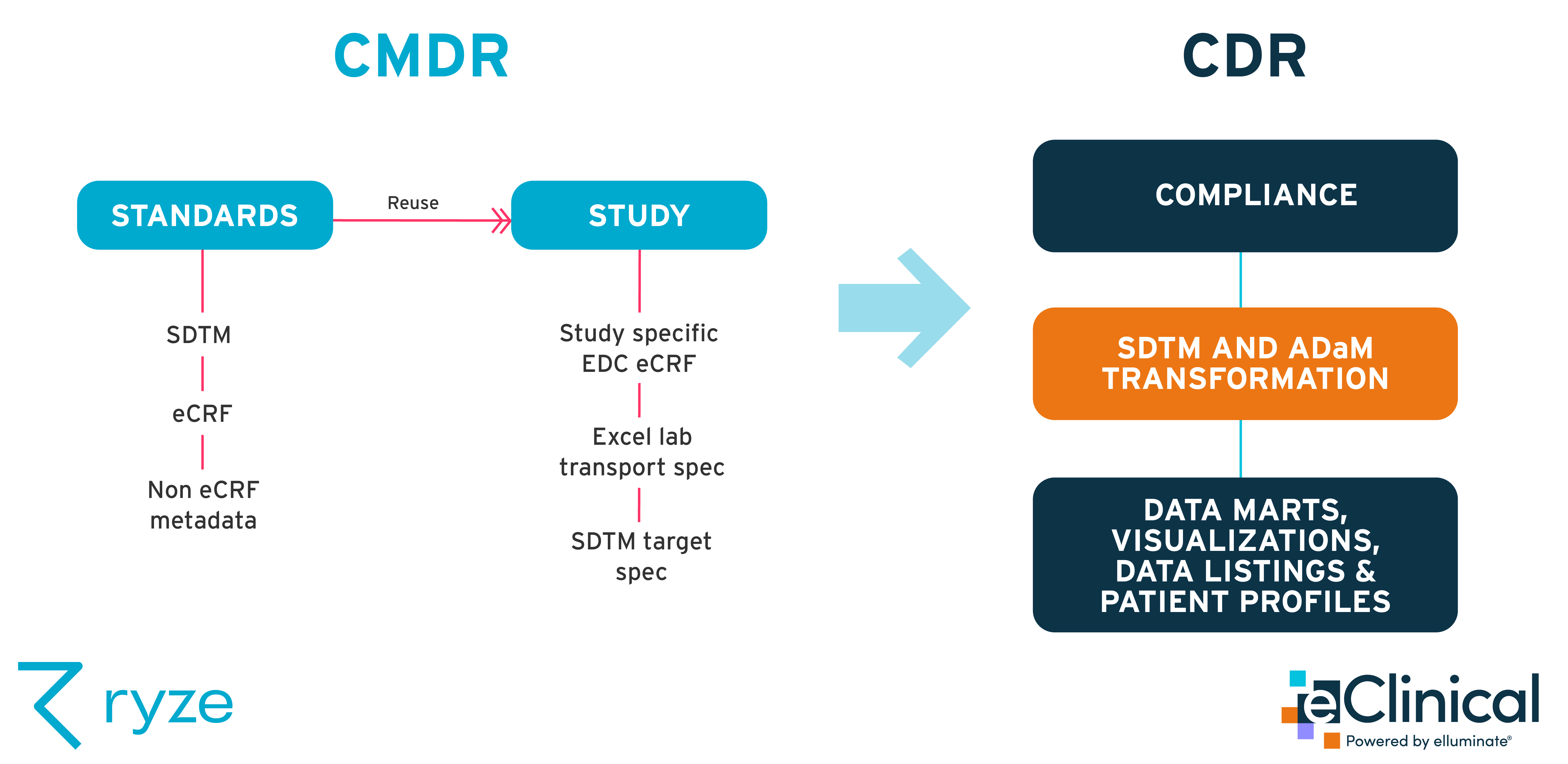
This functionality offered the perfect synergy with our ryze Clinical Metadata Repository (CMDR) and automation platform. ryze lets you standardize and automate clinical study set up and build, meaning you can build CDISC SDTM compliant trials in as little as 6 weeks.
Our ryze CMDR and SDTM conversion engine, together with elluminate’s clinical data ingestion, reporting and analysis platform, provides an end-to-end solution that’s ready to go NOW. We want to empower and inform pharmas, biotechs and CROs with instant access to data insights, and faster decision-making abilities.
Powered by APIs
This powerful integration of two best-in-breed platforms is exactly what our API facilitates. We gave eClinical Solutions access to our open APIs. It’s this code that enables communication between our ryze platform and the elluminate suite. And our APIs are robust and advanced enough to work straightaway, with very little customisation required.
Benefits of a CMDR-CDR integration
There are many deeper benefits you can draw from a CMDR / CDR technology integration, on top of the singular benefits delivered by each system. These include:
- Quickly & easily find specs. You can instantly find spec requirements for any vendor.
- Reuse and automatically generate specs, such as lab transfer specs. Our integration allows the machine-readable part to be used downstream in the study.
- Reduced manual effort. There’s no need to create specs manually in Word or Excel, so you’ll see a big decrease in manual speccing.
- Automatically check data against your original specifications. For example, once you receive patient data, you can check that it complies with your specs in real time, and make sure that your studies adhere to specs.
- Improved compliance and data quality, from study set up through to analysis. Since lab specs are auto-generated, there’s less chance of manual errors. Plus, you can see any issues or errors immediately.
- Faster data insights - even daily data insights if needed. No lag time.
- You can use multiple EDCs (even for different study phases) due to our integration with multiple EDC systems.
See our integration in action
Want to see it for real? The ryze-elluminate integration is ready to go! No smoke and mirrors. You can request a demo tailored to your specific requirements or areas of interest.
If you want to learn more, our joint webinar shows thought leaders from Formedix and eClinical Solutions discussing metadata management, study automation, and ultimately how to gain faster insights with Clinical Metadata and Data Repositories. And it concludes with a demo of the ryze-elluminate integration, so you can see what it could do for your organization.
You can also read more about our partnership with eClinical Solutions in our blog post ➡️ Formedix Partners with eClinical Solutions.
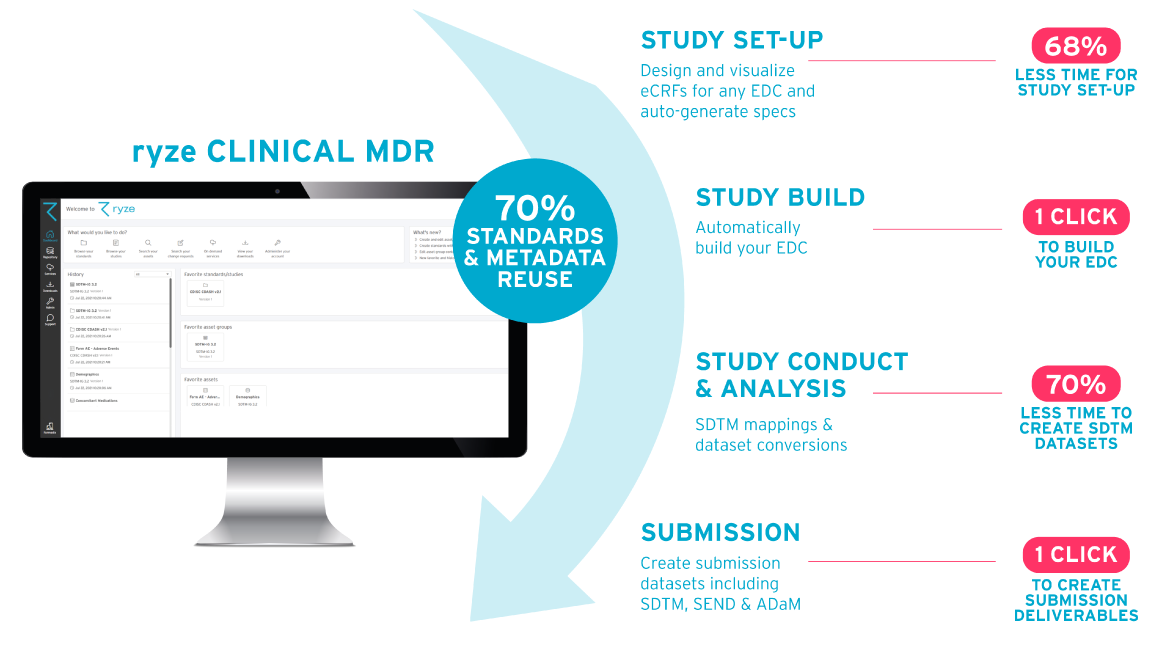
ryze Clinical MDR and automation suite
In 2021 Formedix launched ryze, our off-the-shelf cloud-based Clinical MDR and study automation suite. ryze provides the tools for organizations to leverage faster, more efficient, and higher quality trials, by standardizing and automating clinical study setup and execution. ryze enables the rapid set-up of trials, in compliance with the requirements of the Clinical Data Interchange Standards Consortium (CDISC).
- Easily manage standards & studies in one central library
- Design & build studies for leading EDCs
- See how CRFs look for leading EDCs before building your study
- Automatically comply with latest & previous CDISC standards
- Automate EDC builds & SDTM conversions
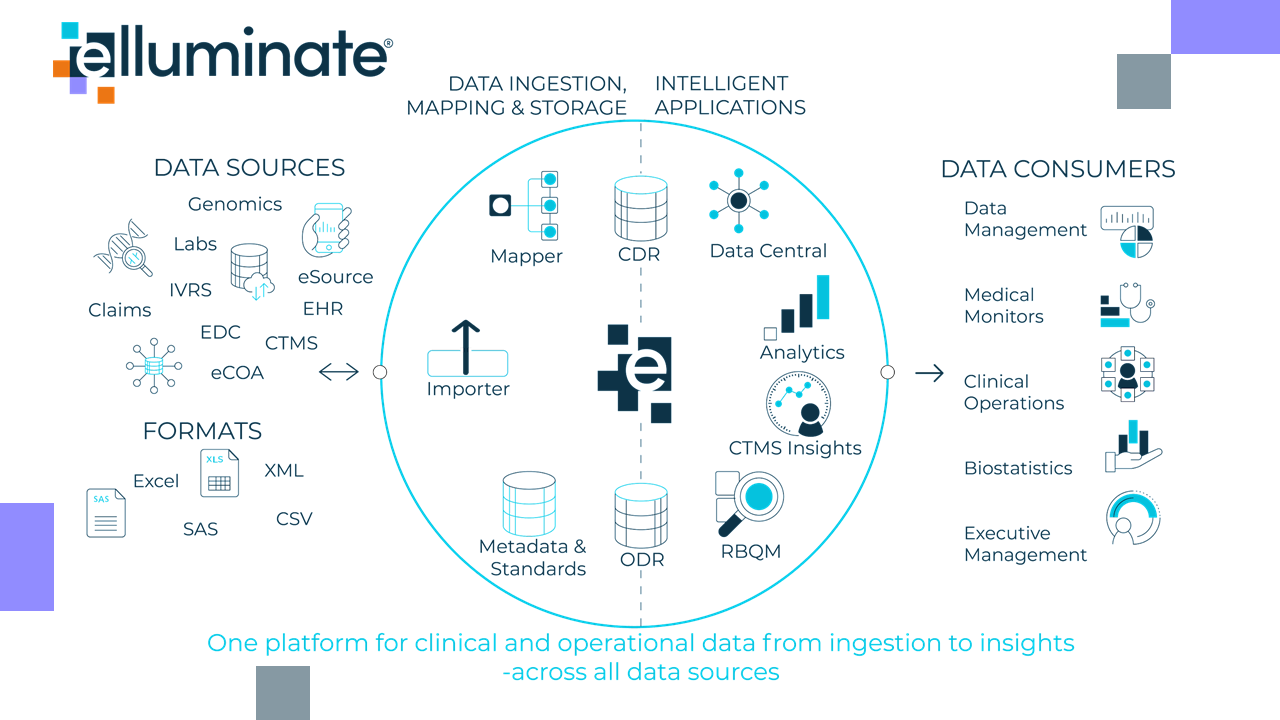
eClinical's elluminate
The elluminate platform from eClinical Solutions maximizes the value of clinical data with faster insights from data acquisition through analytics. A best-in-class CDR gives you an end-to-end platform optimized for common use cases, with:
- Faster access to data and earlier insights
- Increased control with one single source of truth
- Reduced cycle times
- Improved quality throughout the clinical lifecycle
elluminate’s customers use data driven insights to deliver operational oversight and control over high-volumes of biomarker samples.
Book a Demo to see the ryze-elluminate integration LIVE!!!












