The fire and ice of oncolytic virotherapy

In the language of immuno-oncology (IO), the terms “hot” and “cold” refer to tumours that are inflamed or non-inflamed, respectively. “Hot” tumours tend to respond well to IO therapies like checkpoint inhibitors (Cis), while “cold” tumours tend not to respond at all. Unfortunately, most tumours are “cold”, with limited immune cell presence or infiltration, and it is for this reason that only an estimated 12% of patients benefit from CIs when all is said and done.1
Let that sink in. 12% is an incredibly low number, especially in comparison with chemotherapy, where response rates – taking into consideration all tumour types –average around 48.6%.2 Moreover, even in “hot” tumours like non-small cell lung cancer (NSCLC) or melanoma, which are known to benefit from checkpoint inhibitors, resistance to CIs most often sets in after an initial response.
And so, the search is on to find combination therapies that turn tumours that are cold and CI-insensitive, into hot and CI-sensitive ones. This article focuses on one of those potential combination therapies: oncolytic viruses (OVs).
Ice
In the tug-of-war between fire (immune stimulation) and ice (immune suppression), ice usually wins out, since most tumours - with the notable exceptions of melanoma and non-small cell lung cancer (NSCLC) - are “cold” or non-immunogenic. Therapies called checkpoint inhibitors (CI), with names like Opdivo (nivolumab) and Keytruda (pembrolizumab) that are familiar from ubiquitous TV ads - (“A chance to live longer, with Opdivo”) - have the potential to cure end-stage cancer, but only in around 12% of patients with hot or immune-inflamed tumours.3
Unfortunately, the other 88% of patients do not benefit from CIs, which is grossly unfair, to say the least.
To remedy this iniquity, an intensive search is on to find therapies which reverse or relieve immune suppression and make checkpoint inhibitors more active and effective, so that a majority of patients benefit from them.
In addition, these therapies must not increase the autoimmune-like toxicities – some of them severe and fatal – that are common with checkpoint inhibitors.
Fire
One class of therapies that seems to perfectly fit this bill is oncolytic viruses (OVs), which I work with regularly. Many oncolytic viruses, meaning that they preferentially infect and dissolve or lyse tumour cells, are armed with a piece of DNA called a transgene that is intended to further ‘fire up’ the immune system, and, in return - hopefully - the combination of the immunostimulatory virus and its immunostimulatory transgene makes cold or non-treatment responsive tumours hot or treatment responsive.3
Two Ovs have received FDA approval. The first is talimogene laherperepvec, or T-VEC, a modified herpesvirus that was approved in 2015 for the treatment of localised melanoma.
The second is nadofaragene firadenovec, or Adstiladrin, a modified adenovirus that was approved for the treatment of bladder cancer.
Of the several types of therapies and immunotherapies that have already been combined with checkpoint inhibitors, including other checkpoint inhibitors, chimeric antigen receptor (CAR)-T cells, cytokines, radiation, chemotherapy, targeted agents, antibodies, epigenetic inhibitors, and antitumour vaccines - all, by and large, increase activity, but at the expense of significantly more toxicity. This means that their use together is currently limited or off the table altogether.
In contrast, because OVs like T-VEC and Adstiladrin are minimally toxic, their approvals open the door to a brighter future in which they are used regularly together with checkpoint inhibitors to make tumours hotter and CIs more active, as shown below.
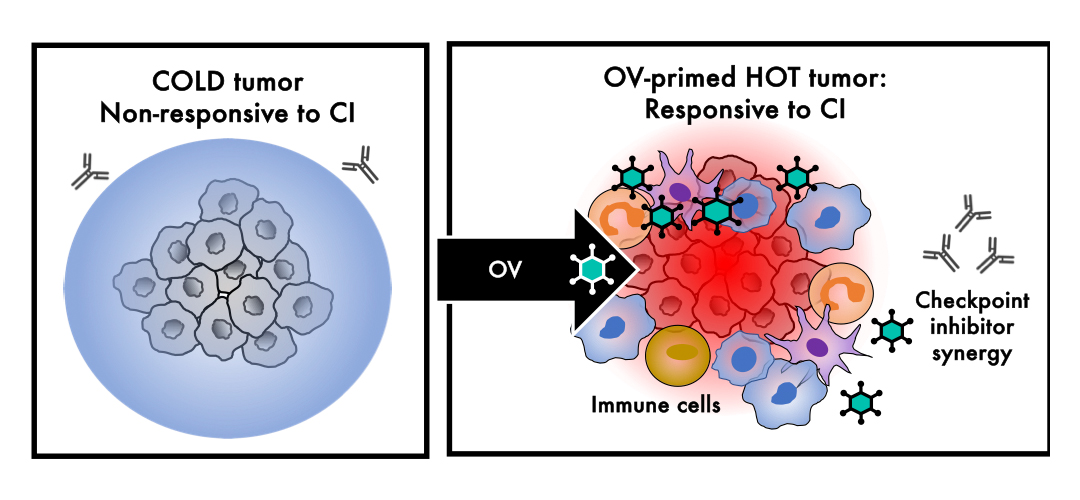
Like Houdini, cancer is a master escape artist with a Plan A, B, C, D, and E already in place, which limits the potential benefit of one single therapy - no matter how active or potent that one therapy is - because eventually cancer finds a way out. However, the use of therapies in combination, each of which may block different possible escape routes and back-up plans, makes it easier or more likely to contain, control, or possibly even cure cancer, provided that these combinations are not too hard-to-tolerate for patients. Conceptually, this is like the cocktail of therapies used for HIV, which transformed it from a virtual death sentence to a manageable chronic disease, and, hopefully, will transform incurable metastatic cancer along the same lines.
Conclusion
For all the hope and hype that surround checkpoint inhibitors, and they have unquestionably revolutionised the treatment of cancer with the promise of durable responses and even cures in some cases, the fact remains that CIs only benefit a subset of patients in a subset of “hot” or immune responsive tumours.
Combination of CIs with several approved classes of therapies like other CIs and chemotherapy are, in principle, attractive and feasible, but the reality is that, except for Ovs, these therapies tend to increase severe side effects. Severe side effects are a reason to reduce the CI dose, or to discontinue CIs permanently, both of which increase the risk of premature death.
The future is perhaps brighter because of the promise of OV and CI combinations in “cold” tumour types, but, as of right now, it is still just a promise. Further clinical trials are needed, which will hopefully prove that OVs make several checkpoint inhibitors better and less toxic for the benefit of all cancer patients, and especially those with cold tumours like gliomas, ovarian, prostate, and pancreatic cancers that do not typically benefit from them at all.
References
- Haslam A et al. Estimation of the Percentage of US Patients With Cancer Who Are Eligible for Immune Checkpoint Inhibitor Drugs. JAMA Netw Open. 2020 Mar 2;3(3):e200423. doi: 10.1001/jamanetworkopen.2020.0423.
- Maldonado EB et al. Estimation of US patients with cancer who may respond to cytotoxic chemotherapy. Future Sci OA. 2020 May 25;6(8):FSO600. doi: 10.2144/fsoa-2020-0024.
- Larson C et al. Commentary on oncolytic viruses: past, present, and future. J Immunother Cancer. 2023 Dec 21;11(12):e007905. doi: 10.1136/jitc-2023-007905.












