Tarsus gets first FDA nod for mite blepharitis therapy
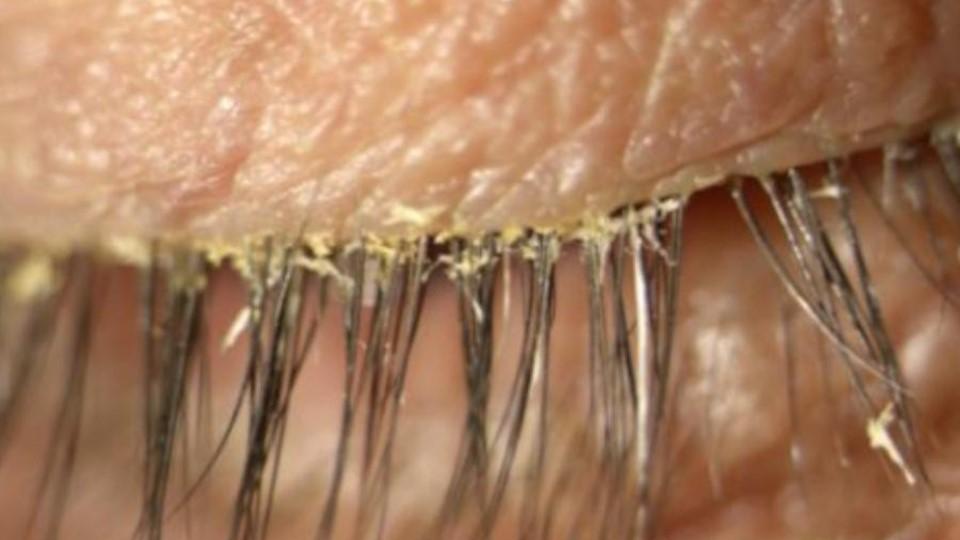
A common eyelid disease caused by infestation with tiny parasitic mites that live in hair follicles now has its first approved treatment in the US.
Tarsus Pharmaceuticals' Xdemvy (lotilaner) has become the first FDA-approved therapy for Demodex blepharitis, a condition that affects up to 25 million people in the US and can lead to red itchy eyes and sometimes the loss of eyelashes.
As alarming as it sounds, Demodex mites are found in just about everyone, and in most cases are harmless and may even contribute to the health of skin by removing dead cells and oil.
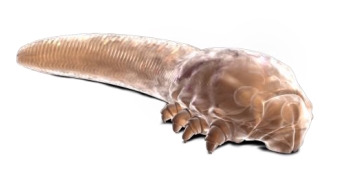
Some people get an overgrowth in mites that can result in problems, and one consequence can be blepharitis, caused by a blockage in oil glands at the base of eyelashes. In severe cases, blepharitis can lead to inflammation of the conjunctiva and damage to the cornea of the eye.
Tarsus' new product is an eyedrop formulation of lotilaner, a drug that is already widely used to treat fleas and ticks in pets. Formerly known as TP-03, Xdemvy has been approved after reducing signs of Demodex infestation and improving blepharitis symptoms in two phase 3 studies.
In the SATURN-1 and SATURN-2 studies, the drug was shown to significantly reduce the formation of collarettes – dandruff-like deposits consisting of partially digested epithelial cells, mite waste, and eggs that typically form at the eyelash base – and also to reduce redness in the eye.
Tarsus said it plans to launch the product next month and, according to chief executive Bobak Azamian, there could be as many as 1.5 million people in the US seeking treatment for Demodex blepharitis. Tarsus has previously said it would launch the product with a wholesale acquisition price of between $1,500 and $2,000.
Currently, patients with Demodex blepharitis try to manage the condition by daily cleaning of the eye with warm water, compresses to relieve swelling, and home remedies like tea tree oil, which anecdotally can help discourage mites.
Medicated ointments containing drugs like ivermectin, metronidazole, and permethrin are also prescribed in some cases, but have limited data to support their efficacy and aren’t FDA approved for this use.
Tarsus says that Xdemvy is the first drug to offer "potentially complete" disease resolution, and has predicted that sales of the drug could eventually top $1 billion a year, pointing to a survey it commissioned of ophthalmologists and optometrists that found that 93% would prescribe an FDA-approved therapy for the condition.
The company has started direct-to-consumer (DTC) advertising campaigns – "Look at the Lids" and "Don’t Freak Out. Get Checked Out" – to raise awareness that red, itchy, and irritated eyelids may be caused by Demodex.