Tandem's insulin pump can predict and prevent hypo's

New predictive software integrated into an insulin pump can reduce time spent in hypoglycaemia compared with sensor-augmented pumps in type 1 disease.
Medical device firm Tandem Diabetes Care has produced results from its PROLOG trial, which it will submit to the FDA, potentially paving the way for the product's launch this summer.
Manufacturers of insulin pumps are competing to produce 'intelligent' pumps that can monitor and automatically respond to levels of insulin in patients’ blood streams.
Tandem is competing with rivals such as Medtronic, which has produced a hybrid closed-loop system that adjusts basal insulin delivery based on blood glucose measurements, although users still input mealtime doses and calibrate the system.
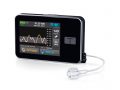
Tandem is hoping that the experimental predictive low glucose suspend (PLGS) feature on its t:slim X2 insulin pump will give it the edge.
The company has been updating features on its pump over the years, allowing patients to improve and tweak their device using software updates.
The PLGS system works by suspending insulin delivery when low blood glucose is predicted, and automatically resuming it when glucose levels begin to rise.
Trial data announced at the Annual Conference on Advanced Technologies and Treatment for Diabetes (ATTD) in Vienna showed the system achieved the primary outcome of reducing time sent in hypoglycaemia compared to sensor-augmented pump therapy alone.
Use of the system during a 3-week period at home reduced the number of sensor glucose readings below 70 mg/dL by 31% compared to the control period using a standard CGM-integrated t:slim X2 Pump without automated insulin suspension.
This marked reduction of time spent in low glucose was accomplished without any increase in the rate of hyperglycemia.
The PROLOG study was a multi-centre, randomised crossover clinical trial comparing two three-week periods of at-home insulin pump use, one period using the t:slim X2 Pump with PLGS, and another period using a standard CGM-integrated t:slim X2 Pump without automated insulin suspension.
The study included 103 participants with type 1 diabetes age 6 to 72 at four research centres across the United States and was co-ordinated by the Jaeb Center for Health Research in Tampa, Florida.
The mean A1C of participants entering the study was 7.3%, with the majority already using a pump (83%) and/or CGM (84%). The primary endpoint of the study was to demonstrate a reduction in the percentage of CGM values below 70 mg/dL when using Tandem’s PLGS feature.
Hypoglycaemia is a huge problem for people being treated for their diabetes and has been linked to increased risk of cardiovascular problems.
The problem is made worse because patients in this state do not realise that they are having a hypoglycaemic episode as their judgement becomes impaired.
[caption id="attachment_37451" align="alignnone" width="120"] Kim Blickenstaff[/caption]
Kim Blickenstaff[/caption]
Kim Blickenstaff, president and CEO of Tandem Diabetes Care, said: “Reducing the risk of hypoglycemia has been the most requested feature of automated insulin delivery in our market research, due to the severity of the complications when left untreated. The data from this study supports our mission to improve the lives of people with diabetes through new innovations, such as the t:slim X2 Pump with Basal-IQ technology.”













