Takeda grabs late-stage haematology drug from Protagonist
Takeda has bolstered its near-term pipeline in rare haematological disorders by licensing rights to a polycythaemia vera (PV) treatment being developed by Protagonist Therapeutics.
The Japanese pharma group is paying $300 million upfront for rights to rusfertide, an injectable hepcidin mimetic currently in the pivotal phase 3 VERIFY trial in PV that could generate preliminary results later this year. The deal includes another $330 million in potential milestone payments plus royalties on sales outside the US.
Takeda has claimed exclusive ex-US rights to rusfertide and will take the lead on commercialising the drug in the US if it gets approved, with Protagonist retaining the right to participate in US sales on a profit-sharing basis. If it opts out, the milestone portion of the deal rises to a maximum of $975 million and the royalty rate goes up to 14% to 29%, according to an SEC filing.
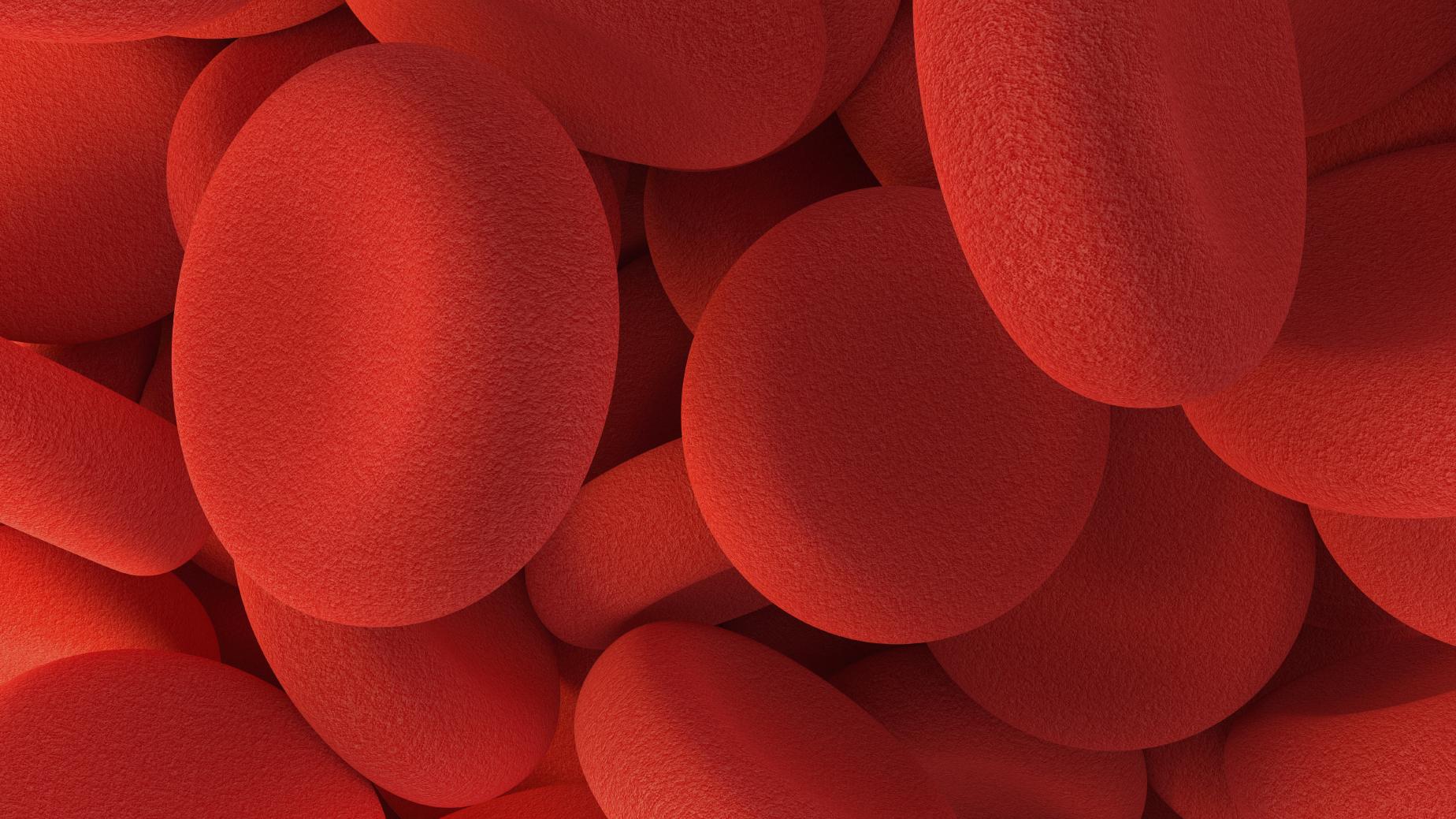
People with PV suffer from an overproduction of red blood cells, which leads to a thickening of the blood and an increased risk of blood clots and affects around 100,000 people in the US alone and a similar number in Europe. The clots can cause serious cardiovascular problems, such as stroke and heart attack, resulting in increased morbidity and mortality.
Patients sometimes have to undergo therapeutic phlebotomy or repeated controlled blood-letting to manage the condition. Drug treatments are also used to treat the disease, but the standard first-line therapies of hydroxyurea or interferons are not particularly effective at controlling blood counts and can be poorly tolerated.
Novartis’ JAK 1/2 inhibitor Jakafi/Jakavi (ruxolitinib), meanwhile, is used as a second-line therapy for refractory patients, but Protagonist has pitched rusfertide at first-line use, as it acts in the same way as the endogenous hepcidin hormone, but has improved pharmacokinetic properties.
Like hepcidin itself, rusfertide is thought to regulate iron homeostasis and control the absorption, storage, and distribution of iron in the body, helping to control the body’s production of red blood cells.
Protagonist's chief executive, Dinesh Patel, described the deal with Takeda as “transformational”, as it will allow the company to focus all its attention on the phase 3 trial, rather than building commercial teams.
“This deal mitigates the inherent execution risks of a first-time commercial launch, optimises the timing, and enhances the scope for peak potential sales of rusfertide,” he said.
For Takeda, rusfertide adds to an emerging portfolio in rare blood disorders currently headed by recently-approved Adzynma (recombinant ADAMTS13) for clotting disorder congenital thrombotic thrombocytopenic purpura (cTTP), which affects fewer than 1,000 patients in the US.
The drug was cleared by the FDA last year, becoming the first alternative to prophylactic plasma-based therapy used to indirectly replenish ADAMTS13 levels in the body.












