Safety restrictions spell the end for Servier osteoporosis drug
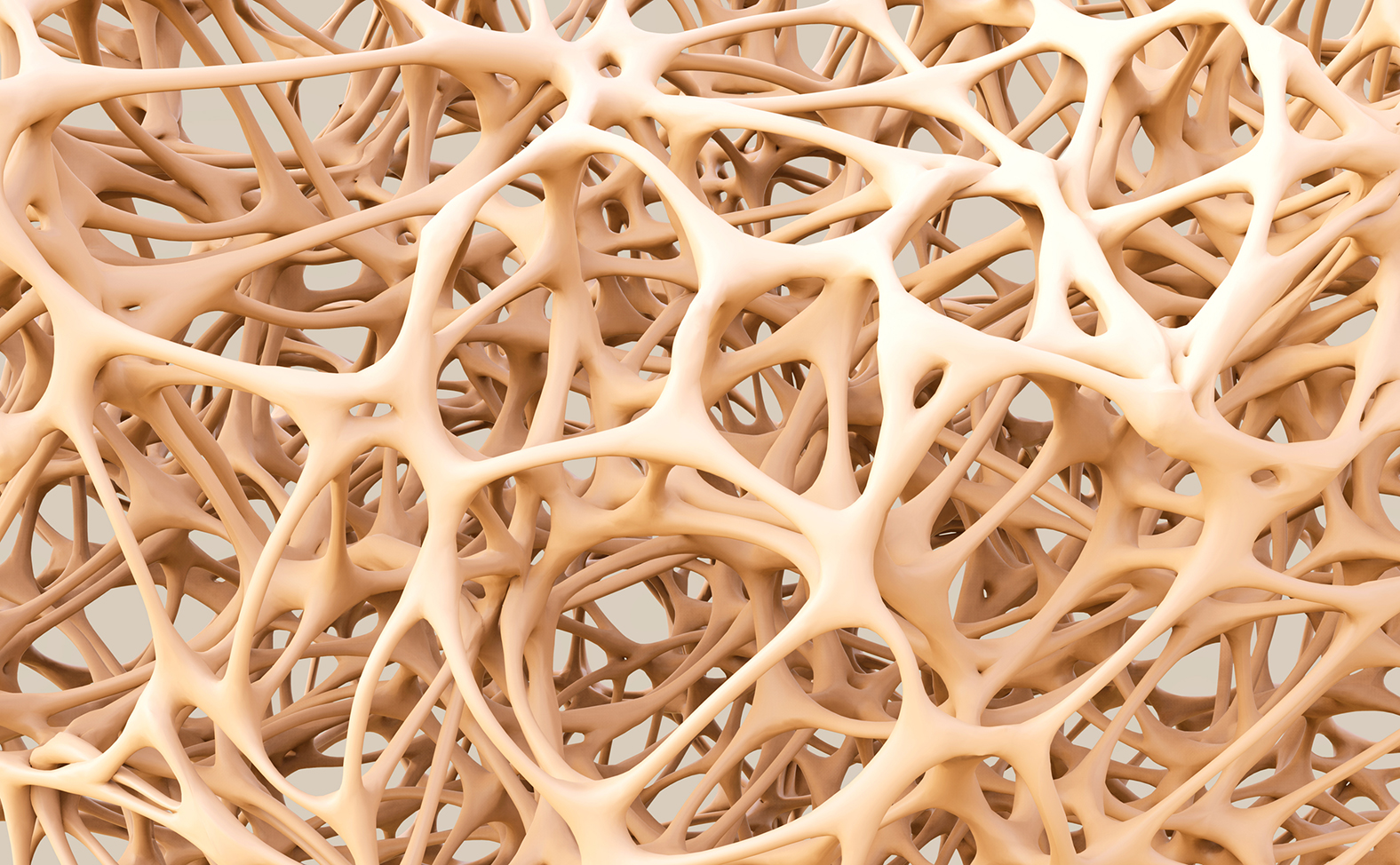
Servier is to abandon its osteoporosis drug, Protelos/Osseor, following safety restrictions imposed by EU regulators three years ago.
The company says sales of Protelos (strontium ranelate) have been in decline since the restrictions were introduced in 2014, and believes it has more promising revenue streams.
In postmenopausal women, Protelos reduces risk of vertebral and hip fractures – but cardiovascular risk has to be managed by restricting its use to patients with no history of heart and circulatory problems, and limiting its use to those who cannot take other osteoporosis drugs.
The European Medicines Agency has ruled that patients treated with the drug should be screened and monitored regularly, every six to 12 months, but this has seen its use decline sharply.
Servier notified European regulators earlier this year that it will cease marketing and supply of the drug, with manufacturing ending in August.
In the UK, Servier has been in touch with patient and professional organisations such as the National Osteoporosis Society, the British Society of Rheumatology and the Primary Care Rheumatology Society.
There are alternative medications on the market for osteoporosis – such as Amgen’s well established Prolia (denosumab).
Radius Health’s Tymlos (abaloparatide) could be approved be European regulators later this year – but Amgen/UCB’s Evenity (romosozumab) has run into trouble with an unexpected cardiac safety signal, delaying a US regulatory decision until next year.












