Second FDA okay for Therapixel's AI-based mammography tool
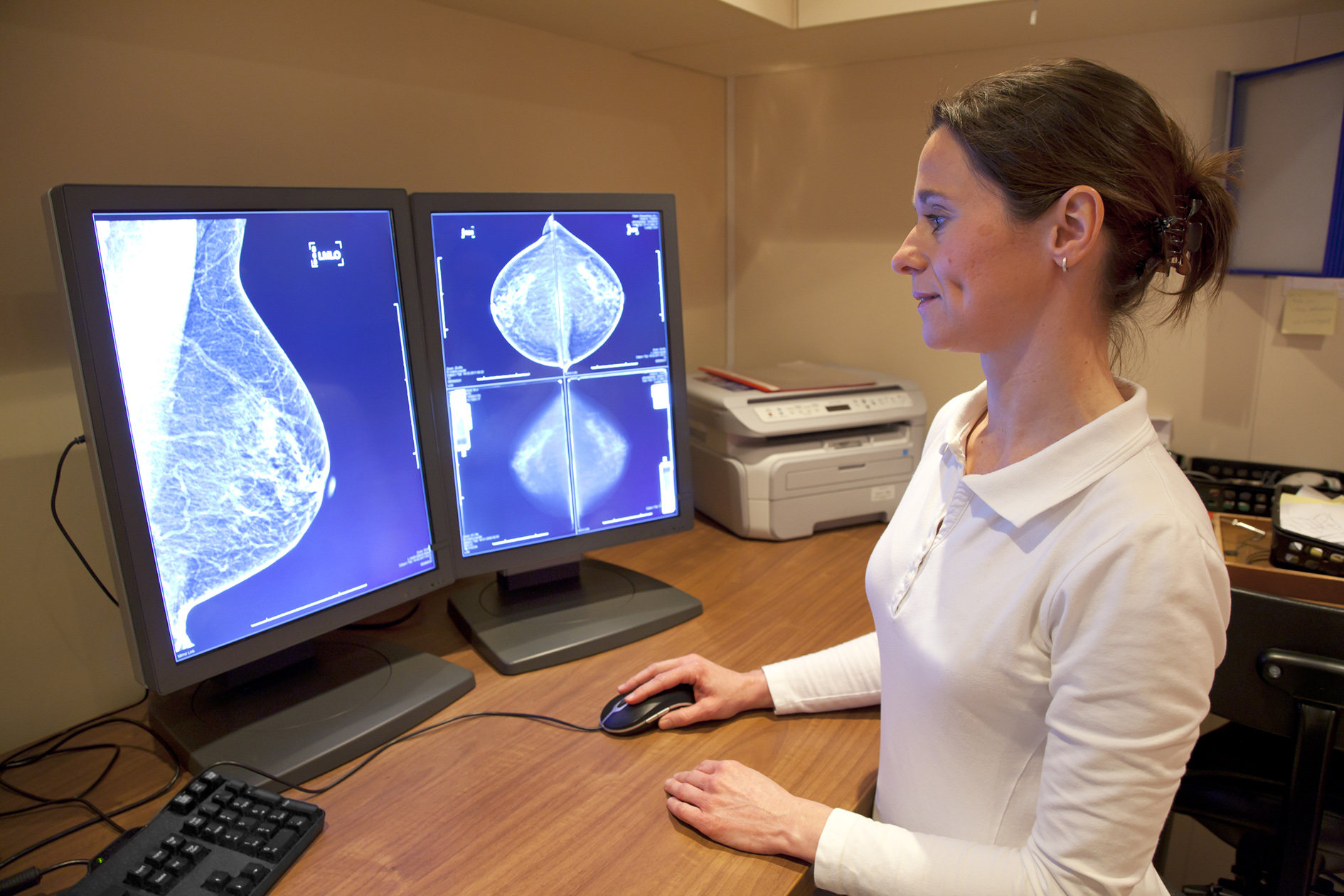
France's Therapixel has secured a second FDA approval for MammoScreen, its artificial intelligence-powered software for detecting cancerous lesions in mammogram images.
MammoScreen was approved last year for use alongside conventional digital 2D mammography, and has now been given a green light by the FDA for use with 3D mammograms, also known as digital breast tomosynthesis.
The software works the same way in both applications, evaluating images to look for suspect lesions and provide "quick and reliable confirmation" for radiologists.
Breast cancer is a leading cause of death among women worldwide and many countries have introduced mammography screening programmes to detect and treat it early.
Examining mammograms for early signs of cancer is high volume, repetitive work for radiologists, and it is well established that some cancers may be missed.
AI software can therefore serve as a backup to avoid some cancers slipping through the net, although a recent study suggested the technology is not yet robust enough to replace humans.
3D mammography is newer but is becoming established as an improved way to look for breast cancer, as it uses multiple images, giving radiologists a clearer picture of the tissue, and raises the chances of a lesion being detected.
It also makes it easier to gauge the size of a cancer, which could have an impact on treatment, and reduces the chances of false positives. However, the increased number of images to review means it is more time-consuming.
Therapixel says MammoScreen automatically detects and characterises suspicious soft tissue lesions and calcifications in mammogram images while assessing their likelihood of malignancy, using a 10-point scale.
It claims clinical testing has shown improvements in readers' performance in cancer detection in mammograms when paired with MammoScreen compared to radiologists alone, and also time savings.
"Receiving this second FDA clearance for MammoScreen is a major milestone for Therapixel," said Pierre Fillard, the company's founder and chief scientific officer.
"MammoScreen can now assist all radiologists in their day-to-day-work, whichever modality they are using," he added.













