Novo puts $285m into monthly GLP-1 based on Ascendis tech
Novo Nordisk has partnered with Ascendis Pharma on less frequently dosed drugs for obesity and other metabolic diseases, starting with a once-monthly GLP-1 receptor agonist.
The Danish pharma group is pledging $285 million in upfront and milestone payments for the lead GLP-1 asset in the partnership, with another $77.5 million apiece on offer for any additional candidates it decides to take forward through development.
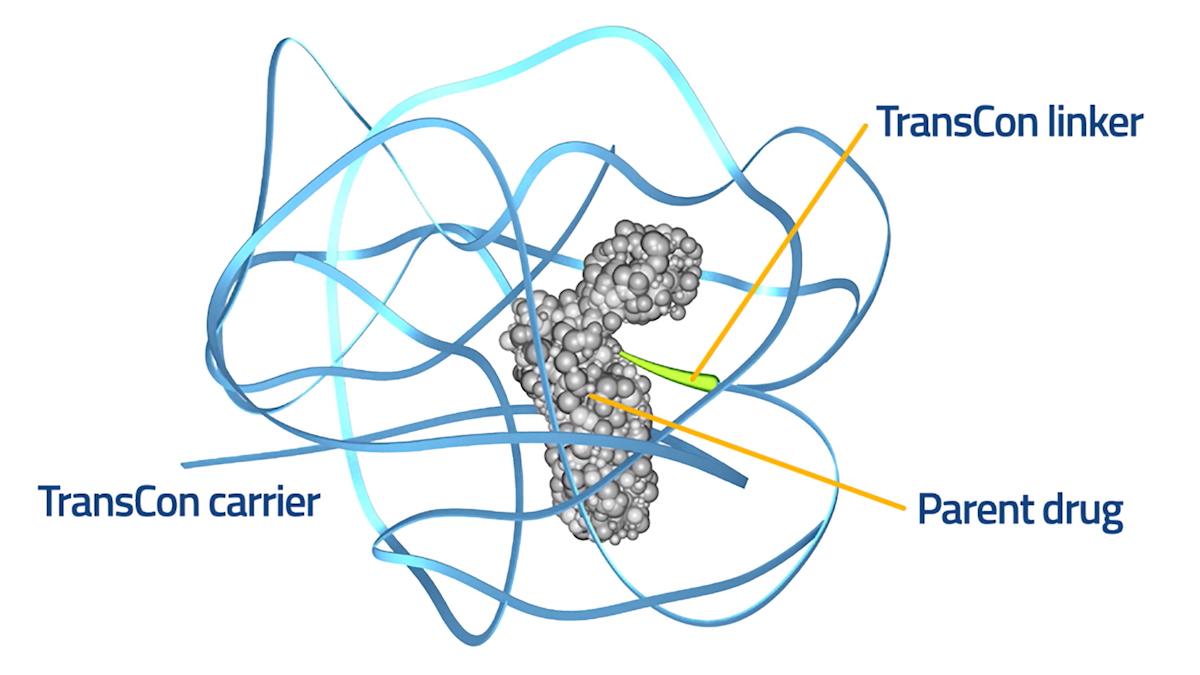
At the heart of the agreement is an exclusive worldwide license to Copenhagen-based Ascendis' TransCon technology platform, which involves adding linker and carrier molecules to turn established medicines into prodrugs with improved delivery characteristics. The prodrugs eventually dissociate to release the active ingredient in a controlled manner, according to Ascendis.
The company has already demonstrated the value of its technology in two approved products, namely Yorvipath (palopegteriparatide), a modified version of parathyroid hormone (PTH), which is for the treatment of hypoparathyroidism in adults, and Skytrofa (lonapegsomatropin), a long-acting growth hormone for children with growth hormone deficiency.
Yorvipath cuts the number of daily injections needed to treat hypoparathyroidism to one from two or three, while Skytrofa is a once-weekly injection that offers an alternative to current therapies that need to be dosed multiple times a week and sometimes daily.
Ascendis is expecting sales of Skytrofa to reach up to €240 million this year, and saw just over €5 million in Yorvipath turnover in the second quarter from initial launches in Germany and Austria. Its third TransCon candidate, meanwhile, is navepegritide, a treatment for children with achondroplasia, which is heading for an FDA filing in the first quarter of 2025.
Partnering with Novo Nordisk could be transformative for the Danish biotech, allowing it to tap into the burgeoning market for GLP-1 therapies for diabetes, obesity, and other associated conditions.
Novo Nordisk's current products like semaglutide-based Ozempic and Wegovy need to be dosed at least once a week. Reducing that frequency could be a competitive advantage against similarly-dosed rivals like Eli Lilly's tirzepatide-based Mounjaro and Zepbound, help patients comply with treatment, and potentially reduce the cost of therapy.
"Developing potential therapies that can be administered less frequently could benefit societies, as well as individual patients, and it is a clear focus area for Novo Nordisk," said Brian Vandahl, the company's head of global research technologies.
Under the terms of the alliance, Ascendis will carry out the early development of TransCon product candidates, funded by Novo Nordisk, with the pharma group taking over once they reach the clinical development stage.












