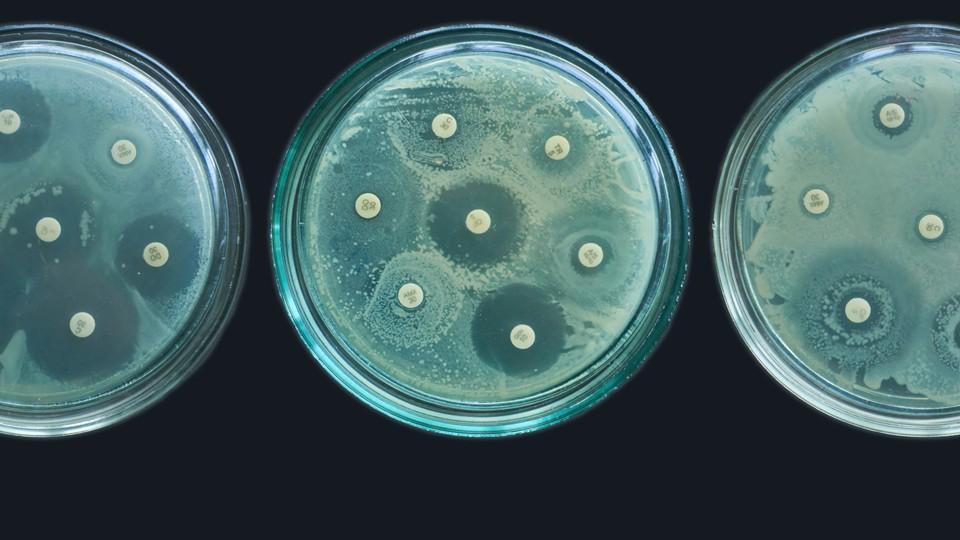
NHS may double subscription rate for novel antibiotics
The yearly subscription-style payments made by NHS England to developers of novel antibiotics could be doubled from £10 million to £20 million in some cases, according to proposals aimed at expanding the scope of the programme.
A consultation on the future direction of the ‘Netflix-like’ pilot scheme has been launched, almost exactly a year after the first two contracts for antibiotics were given to Shionogi and Pfizer.
Under the scheme, the NHS pays a fixed annual fee for access to the two medicines, irrespective of how much of the medicine is actually used to treat patients.
The hope is that separating the amount of antibiotic used from the payment model could entice pharma companies – which have abandoned antimicrobial R&D in their droves – to enter the category once again and help to develop new drugs that will tackle the pressing issue of antimicrobial resistance (AMR).
The proposal to increase the top payment rate to £20 million would apply to new drugs that offer “outstanding clinically based criteria” and “exceptional value to patients and taxpayers,” said NHS England.
It noted the criteria that will be used has been developed by health technology assessment (HTA) agency NICE, based on the experience gained in the pilot to date, and the new consultation will also evaluate new products that may be included, focusing on drugs that are active against pathogens on the World Health Organization (WHO) priority list.
A Biotechnology Innovation Organization (BIO) report published last year concluded that the current antibacterial pipeline – hit by a massive retreat by big pharma companies from the category in recent years – is simply inadequate to meet the growing threat of antibiotic-resistant pathogens.
The lack of investment reflects the widely-accepted view that the market for antibiotics is broken, with new agents reserved for last-line use when older drugs are ineffective, and therefore generating limited revenues to offset their R&D costs. That has also meant that smaller biopharma companies operating in this area have struggled to build a commercially viable business.
Commenting on the development, the chief executive of the Association of the British Pharmaceutical Industry (ABPI), Richard Torbett, said: “The UK is leading this fight by developing a pragmatic new approach to help secure the antibiotics the world needs.”
So far, the UK remains the pioneer of the subscription payment model, but there are signs of activity in other countries. That includes the US, where the Pioneering Antimicrobial Subscriptions to End Upsurging Resistance (PASTEUR) Act – reintroduced in the House and Senate in April – could introduce a similar approach if enacted.