Musk's Neuralink close to clinical trials of "brain interface" device
A new brain implant technology from Elon Musk’s tech startup Neuralink could be used as a treatment for neurologic conditions and Parkinson’s disease.
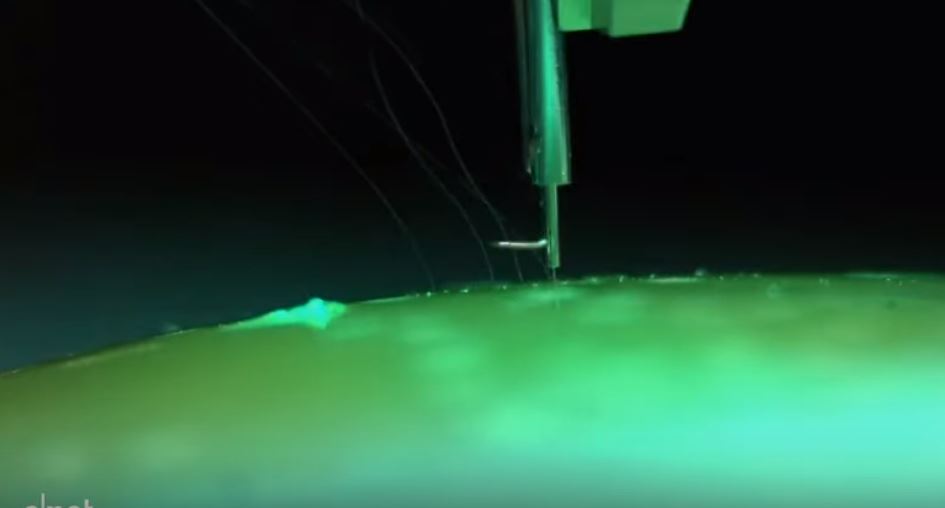
Musk describes Neuralink as a “brain-machine interface” company - and its latest invention is based on “threads” around a thousand times thinner than a human hair that could be injected by a robot into the brain to detect the activity of neurons.
Musk said at a press event, also streamed online, that one goal of the technology is to stave off the “existential threat” of artificial intelligence surpassing human intelligence.
Musk has argued for several years that even if AI is benign, there is a need for a direct interface with a machine to allow humans to keep up with their development.
So far Neuralink’s technology has only been tested in rats and monkeys – Musk has said that the system has allowed a monkey to “control a computer with his brain.”
But according to Neuralink’s president Max Hodak, the technology is close to clinical trials in neurological disorders.
The company will seek FDA approval for a human clinical trial as early as next year in a specially modified version of the device.
This first trial will target patients with complete paralysis due to a break in the upper spinal cord and will involve installing four of its devices in patients’ brains.
There are other potential applications too, such as for Parkinson’s disease, where it could improve on existing deep brain stimulation therapy.
Neuralink thinks the device could be a step forward on existing technology in this field as it would allow 1,000 times more electrodes to interface with the brain than the best FDA-approved device.
Neuralink also thinks that its device will be less risky than current deep brain stimulation techniques, which involve small holes being drilled in the skull.
Musk said at the press event this week: “This has a very good purpose, which is to cure diseases, and ultimately secure humanity’s future.”












